- Record: found
- Abstract: found
- Article: not found
Clearance of the mutant androgen receptor in motoneuronal models of spinal and bulbar muscular atrophy ☆
Read this article at
Abstract
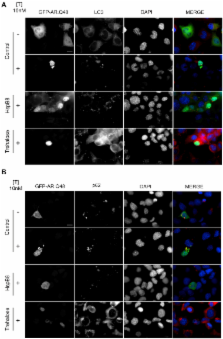
Spinal and bulbar muscular atrophy (SBMA) is an X-linked motoneuron disease caused by an abnormal expansion of a tandem CAG repeat in exon 1 of the androgen receptor (AR) gene that results in an abnormally long polyglutamine tract (polyQ) in the AR protein. As a result, the mutant AR (ARpolyQ) misfolds, forming cytoplasmic and nuclear aggregates in the affected neurons. Neurotoxicity only appears to be associated with the formation of nuclear aggregates. Thus, improved ARpolyQ cytoplasmic clearance, which indirectly decreases ARpolyQ nuclear accumulation, has beneficial effects on affected motoneurons. In addition, increased ARpolyQ clearance contributes to maintenance of motoneuron proteostasis and viability, preventing the blockage of the proteasome and autophagy pathways that might play a role in the neuropathy in SBMA. The expression of heat shock protein B8 (HspB8), a member of the small heat shock protein family, is highly induced in surviving motoneurons of patients affected by motoneuron diseases, where it seems to participate in the stress response aimed at cell protection. We report here that HspB8 facilitates the autophagic removal of misfolded aggregating species of ARpolyQ. In addition, though HspB8 does not influence p62 and LC3 (two key autophagic molecules) expression, it does prevent p62 bodies formation, and restores the normal autophagic flux in these cells. Interestingly, trehalose, a well-known autophagy stimulator, induces HspB8 expression, suggesting that HspB8 might act as one of the molecular mediators of the proautophagic activity of trehalose. Collectively, these data support the hypothesis that treatments aimed at restoring a normal autophagic flux that result in the more efficient clearance of mutant ARpolyQ might produce beneficial effects in SBMA patients.
Related collections
Most cited references79
- Record: found
- Abstract: found
- Article: not found
Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction.
- Record: found
- Abstract: found
- Article: not found
Guidelines for the use and interpretation of assays for monitoring autophagy.
- Record: found
- Abstract: found
- Article: not found
