- Record: found
- Abstract: found
- Article: found
Oligodendrocyte Precursor Cells Modulate the Neuronal Network by Activity-Dependent Ectodomain Cleavage of Glial NG2
Read this article at
Abstract
Abstract
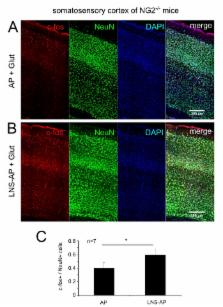
The role of glia in modulating neuronal network activity is an important question. Oligodendrocyte precursor cells (OPC) characteristically express the transmembrane proteoglycan nerve-glia antigen 2 (NG2) and are unique glial cells receiving synaptic input from neurons. The development of NG2+ OPC into myelinating oligodendrocytes has been well studied, yet the retention of a large population of synapse-bearing OPC in the adult brain poses the question as to additional functional roles of OPC in the neuronal network. Here we report that activity-dependent processing of NG2 by OPC-expressed secretases functionally regulates the neuronal network. NG2 cleavage by the α-secretase ADAM10 yields an ectodomain present in the extracellular matrix and a C-terminal fragment that is subsequently further processed by the γ-secretase to release an intracellular domain. ADAM10-dependent NG2 ectodomain cleavage and release (shedding) in acute brain slices or isolated OPC is increased by distinct activity-increasing stimuli. Lack of NG2 expression in OPC (NG2-knockout mice), or pharmacological inhibition of NG2 ectodomain shedding in wild-type OPC, results in a striking reduction of N-methyl-D-aspartate (NMDA) receptor-dependent long-term potentiation (LTP) in pyramidal neurons of the somatosensory cortex and alterations in the subunit composition of their α-amino-3-hydroxy-5-methyl-4-isoxazolepr opionicacid (AMPA) receptors. In NG2-knockout mice these neurons exhibit diminished AMPA and NMDA receptor-dependent current amplitudes; strikingly AMPA receptor currents can be rescued by application of conserved LNS protein domains of the NG2 ectodomain. Furthermore, NG2-knockout mice exhibit altered behavior in tests measuring sensorimotor function. These results demonstrate for the first time a bidirectional cross-talk between OPC and the surrounding neuronal network and demonstrate a novel physiological role for OPC in regulating information processing at neuronal synapses.
Author Summary
Although glial cells substantially outnumber neurons in the mammalian brain, much remains to be discovered regarding their functions. Among glial cells, oligodendrocyte precursors differentiate into oligodendrocytes, whose function is to enwrap nerves with myelin to ensure proper impulse conduction. However, oligodendrocyte precursors also comprise a stable population in all major regions of the adult brain, making up around 5% of the total number of neurons and glia. Synapses are classically formed between neurons. Nonetheless, oligodendrocyte precursors are unique among glial cells in that they receive direct synaptic input from different types of neurons; whether OPC also send signals to neurons is still unknown. Here we show a bidirectional communication between neurons and oligodendrocyte precursors: neuronal activity regulates the cleavage of a glial membrane protein and the release of an extracellular domain that in turn modulates synaptic transmission between neurons. Our data thus show that a particular subtype of glial cells, oligodendrocyte precursors, functionally integrate into the neuronal network and we link this bidirectional signaling to mouse behavior and disease.
Related collections
Most cited references79
- Record: found
- Abstract: found
- Article: not found
NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS.
- Record: found
- Abstract: found
- Article: not found
Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus.
- Record: found
- Abstract: found
- Article: not found