- Record: found
- Abstract: found
- Article: found
The Anti-Inflammatory and Antioxidant Properties of n-3 PUFAs: Their Role in Cardiovascular Protection
Read this article at
Abstract
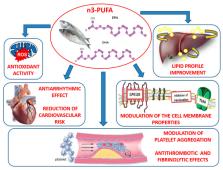
Polyunsaturated fatty acids (n-3 PUFAs) are long-chain polyunsaturated fatty acids with 18, 20 or 22 carbon atoms, which have been found able to counteract cardiovascular diseases. Eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), in particular, have been found to produce both vaso- and cardio-protective response via modulation of membrane phospholipids thereby improving cardiac mitochondrial functions and energy production. However, antioxidant properties of n-3 PUFAs, along with their anti-inflammatory effect in both blood vessels and cardiac cells, seem to exert beneficial effects in cardiovascular impairment. In fact, dietary supplementation with n-3 PUFAs has been demonstrated to reduce oxidative stress-related mitochondrial dysfunction and endothelial cell apoptosis, an effect occurring via an increased activity of endogenous antioxidant enzymes. On the other hand, n-3 PUFAs have been shown to counteract the release of pro-inflammatory cytokines in both vascular tissues and in the myocardium, thereby restoring vascular reactivity and myocardial performance. Here we summarize the molecular mechanisms underlying the anti-oxidant and anti-inflammatory effect of n-3 PUFAs in vascular and cardiac tissues and their implication in the prevention and treatment of cardiovascular disease.
Related collections
Most cited references108

- Record: found
- Abstract: found
- Article: found
Reperfusion injury and reactive oxygen species: The evolution of a concept☆
- Record: found
- Abstract: found
- Article: not found
Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease.
- Record: found
- Abstract: found
- Article: not found