- Record: found
- Abstract: found
- Article: found
Recent advances in antiemetics: new formulations of 5HT 3-receptor antagonists
Read this article at
Abstract
Purpose
To discuss new therapeutic strategies for chemotherapy-induced nausea and vomiting (CINV) involving 5-hydroxytryptamine type 3 (5HT 3)-receptor antagonists (RAs).
Summary
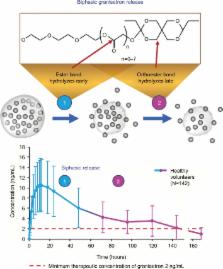
CINV remains poorly controlled in patients receiving moderately emetogenic chemotherapy (MEC) or highly emetogenic chemotherapy (HEC); nausea and delayed-phase CINV (24–120 hours after chemotherapy) are the most difficult to control. National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) antiemesis-guideline recommendations for HEC include a four-drug regimen (5HT 3 RA, neurokinin 1 [NK 1] RA, dexamethasone, and olanzapine). For some MEC regimens, a three-drug regimen (5HT 3 RA, NK 1 RA, and dexamethasone) is recommended. While 5HT 3 RAs have dramatically improved CINV in the acute phase (0–24 hours after chemotherapy), their efficacy declines in the delayed phase. Newer formulations have been developed to extend 5HT 3-RA efficacy into the delayed phase. Granisetron extended-release subcutaneous (GERSC), the most recently approved 5HT 3 RA, provides slow, controlled release of therapeutic granisetron concentrations for ≥5 days. GERSC is included in the NCCN and ASCO guidelines for MEC and HEC, with NCCN-preferred status for MEC in the absence of an NK 1 RA. Efficacy and safety of 5HT 3 RAs in the context of guideline-recommended antiemetic therapy are reviewed.
Conclusion
Recent updates in antiemetic guidelines and the development of newer antiemet-ics should help mitigate CINV, this dreaded side effect of chemotherapy. GERSC, the most recently approved 5HT 3-RA formulation, is indicated for use with other antiemetics to prevent acute and delayed nausea and vomiting associated with initial and repeat courses of MEC and anthracycline–cyclophosphamide combination-chemotherapy regimens.
Related collections
Most cited references113
- Record: found
- Abstract: not found
- Article: not found
The serotonin syndrome.
- Record: found
- Abstract: found
- Article: not found
Antiemetics: American Society of Clinical Oncology clinical practice guideline update.
- Record: found
- Abstract: found
- Article: not found