- Record: found
- Abstract: found
- Article: found
Coagulation during elective neurosurgery with hydroxyethyl starch fluid therapy: an observational study with thromboelastometry, fibrinogen and factor XIII

Read this article at
Abstract
Background
Several studies have described hypercoagulability in neurosurgery with craniotomy for brain tumor resection. In this study, hydroxyethyl starch (HES) 130/0.42 was used for hemodynamic stabilization and initial blood loss replacement. HES can induce coagulopathy with thromboelastographic signs of decreased clot strength. The aim of this study was to prospectively describe perioperative changes in coagulation during elective craniotomy for brain tumor resection with the present fluid regimen.
Methods
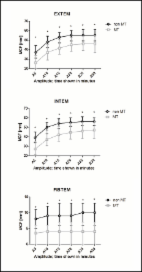
Forty patients were included. Perioperative whole-blood samples were collected for EXTEM and FIBTEM assays on rotational thromboelastometry (ROTEM) and plasma fibrinogen analysis immediately before surgery, after 1 L of HES infusion, at the end of surgery and in the morning after surgery. Factor (F)XIII activity, thrombin-antithrombin complex (TAT) and plasmin-α2-antiplasmin complex (PAP) were analysed in the 25 patients receiving ≥1 L of HES.
Results
Most patients (37 of 40) received HES infusion (0.5–2 L) during surgery. Preoperative ROTEM clot formation/structure, plasma fibrinogen and FXIII levels were generally within normal range but approached a hypocoagulant state during and at end of surgery. ROTEM variables and fibrinogen levels, but not FXIII, returned to baseline levels in the morning after surgery. Low perioperative fibrinogen levels were common. TAT levels were increased during and after surgery. PAP levels mostly remained within the reference ranges, not indicating excessive fibrinolysis. There were no differences in ROTEM results and fibrinogen levels in patients receiving <1 L HES and ≥1 L HES.
Conclusions
Only the increased TAT levels indicated an intra- and postoperative activation of coagulation. On the contrary, all other variables deteriorated towards hypocoagulation but were mainly normalized in the morning after surgery. Although this might be an effect of colloid-induced coagulopathy, we found no dose-dependent effect of HES. The unactivated fibrinolysis indicates that prophylactic use of tranexamic acid does not seem warranted under normal circumstances in elective neurosurgery. Individualized fluid therapy and coagulation factor substitution is of interest for future studies.
Related collections
Most cited references36

- Record: found
- Abstract: found
- Article: found
Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis
- Record: found
- Abstract: found
- Article: found
FIBTEM provides early prediction of massive transfusion in trauma
- Record: found
- Abstract: found
- Article: not found