- Record: found
- Abstract: found
- Article: found
Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis
Read this article at
Abstract
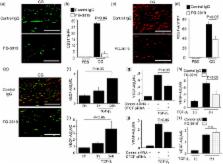
Peritoneal fibrosis (PF) is a serious complication in various clinical settings, but the mechanisms driving it remain to be fully determined. Connective tissue growth factor (CTGF) is known to regulate fibroblast activities. We therefore examined if CTGF inhibition has anti-fibrotic effects in PF. PF was induced by repetitive intraperitoneal injections of chlorhexidine gluconate (CG) in mice with type I pro-collagen promoter-driven green fluorescent protein (GFP) expression to identify fibroblasts. FG-3019, an anti-CTGF monoclonal antibody, was used to inhibit CTGF. CG-induced PF was significantly attenuated in FG-3019-treated mice. CG challenges induced marked accumulations of proliferating fibroblasts and of myofibroblasts, which were both reduced by FG-3019. Levels of peritoneal CTGF expression were increased by CG challenges, and suppressed in FG-3019-treated mice. FG-3019 treatment also reduced the number of CD31 + vessels and VEGF-A-positive cells in fibrotic peritoneum. In vitro studies using NIH 3T3 fibroblasts and peritoneal mesothelial cells (PMCs) showed that CTGF blockade suppressed TGF-β 1-induced fibroblast proliferation and myofibroblast differentiation, PMC mesothelial-to-mesenchymal transition, and VEGF-A production. These findings suggest that the inhibition of CTGF by FG-3019 might be a novel treatment for PF through the regulation of fibroblast and myofibroblast accumulation and angiogenesis.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases.
- Record: found
- Abstract: found
- Article: not found
The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak.
- Record: found
- Abstract: found
- Article: not found