- Record: found
- Abstract: found
- Article: found
Impact of Bacterial Toxins in the Lungs
Read this article at
Abstract
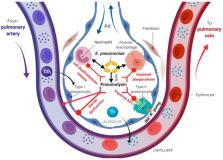
Bacterial toxins play a key role in the pathogenesis of lung disease. Based on their structural and functional properties, they employ various strategies to modulate lung barrier function and to impair host defense in order to promote infection. Although in general, these toxins target common cellular signaling pathways and host compartments, toxin- and cell-specific effects have also been reported. Toxins can affect resident pulmonary cells involved in alveolar fluid clearance (AFC) and barrier function through impairing vectorial Na + transport and through cytoskeletal collapse, as such, destroying cell-cell adhesions. The resulting loss of alveolar-capillary barrier integrity and fluid clearance capacity will induce capillary leak and foster edema formation, which will in turn impair gas exchange and endanger the survival of the host. Toxins modulate or neutralize protective host cell mechanisms of both the innate and adaptive immunity response during chronic infection. In particular, toxins can either recruit or kill central players of the lung’s innate immune responses to pathogenic attacks, i.e., alveolar macrophages (AMs) and neutrophils. Pulmonary disorders resulting from these toxin actions include, e.g., acute lung injury (ALI), the acute respiratory syndrome (ARDS), and severe pneumonia. When acute infection converts to persistence, i.e., colonization and chronic infection, lung diseases, such as bronchitis, chronic obstructive pulmonary disease (COPD), and cystic fibrosis (CF) can arise. The aim of this review is to discuss the impact of bacterial toxins in the lungs and the resulting outcomes for pathogenesis, their roles in promoting bacterial dissemination, and bacterial survival in disease progression.
Related collections
Most cited references310
- Record: found
- Abstract: found
- Article: not found
GsdmD p30 elicited by caspase-11 during pyroptosis forms pores in membranes.

- Record: found
- Abstract: found
- Article: not found