- Record: found
- Abstract: found
- Article: found
Cost-Effectiveness of Earlier Transition to Angiotensin Receptor Neprilysin Inhibitor in Patients With Heart Failure and Reduced Ejection Fraction
Read this article at
Abstract
Background
Angiotensin receptor neprilysin inhibitor (ARNi) therapy improves clinical outcomes in patients with heart failure and reduced left ventricular ejection fraction. However, ARNi therapy uptake remains modest, potentially in part due to perceived cost considerations of early transition from angiotensin converting enzyme inhibitor or angiotensin receptor blocker therapy.
Methods
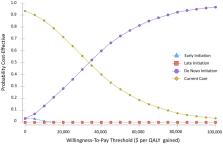
We constructed a decision-analytic Markov model to assess cost-effectiveness of 3 different ARNi initiation strategies according to timing of initiation: (1) de novo, or immediate initiation at baseline, (2) Early or after 3 months, or (3) Late, or after 9 months. Initiation strategies were compared with (4) current care, with utilization of ARNi derived from a large observational database. Total costs, quality-adjusted life-years (QALYs), and the incremental cost-effectiveness ratio (ICER) were estimated over a 5-year time horizon in the base case analysis.
Results
Current care was associated with the lowest total cost (CAD$26,664) and accrued benefit (3.28 QALYs). The de novo strategy yielded an ICER of $34,727 per QALY gained, whereas Early and Late initiation strategies yielded a less favourable ICER per QALY gained of $35,871 and $40,234, respectively. The model was most sensitive to the cost of ARNi therapy.
Résumé
Le traitement par un antagoniste des récepteurs de l’angiotensine/inhibiteur de la néprilysine (ARNI) améliore les résultats cliniques chez les patients présentant une insuffisance cardiaque et une fraction d’éjection ventriculaire gauche réduite. L’adoption d’un tel traitement demeure toutefois modeste, peut-être en partie à cause des perceptions quant au coût associé à la substitution précoce d’un inhibiteur de l’enzyme de conversion de l’angiotensine ou d’un antagoniste des récepteurs de l’angiotensine.
Nous avons mis au point un modèle de Markov appliqué à l’analyse décisionnelle afin d’évaluer le rapport coût-efficacité de trois stratégies d’instauration d’un traitement par un ARNI, selon le moment de la mise en route : 1) instauration de novo, c’est-à-dire instauration immédiate dès le départ; 2) instauration précoce (après trois mois); ou 3) instauration tardive (après neuf mois). Les stratégies d’instauration ont été comparées à 4) la norme de soins actuelle, l’utilisation des ARNI étant dérivée d’une importante base de données observationnelles. Dans l’analyse du scénario de référence, les coûts totaux, les années de vie pondérées par la qualité (QALY pour quality-adjusted life-years) et le rapport coût-efficacité différentiel (RCED) ont été estimés sur une période de cinq ans.
La norme de soins actuelle était associée au coût le plus faible (26 664 $CAD) et à un bienfait cumulé (3,28 QALY). La stratégie de novo a donné lieu à un RCED de 34 727 $ par QALY gagnée, tandis que les RCED des stratégies d’instauration précoce et tardive étaient moins favorables et s’établissaient respectivement à 35 871 $ et à 40 234 $. Le modèle s’est révélé plus sensible au coût du traitement par un ARNI.
La mise en route d’un traitement par un ARNI de novo est attrayante sur le plan financier, et devient de moins en moins intéressante à mesure que le temps passe. Nos résultats indiquent qu’il faudrait instaurer le traitement par un ARNI le plus rapidement possible chez les patients présentant une insuffisance cardiaque et une fraction d’éjection ventriculaire gauche réduite.
Related collections
Most cited references33
- Record: found
- Abstract: not found
- Article: not found
2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC.
- Record: found
- Abstract: found
- Article: not found
Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction
- Record: found
- Abstract: found
- Article: not found