- Record: found
- Abstract: found
- Article: found
LOXL1 modulates the malignant progression of colorectal cancer by inhibiting the transcriptional activity of YAP
Read this article at
Abstract
Background
LOX-like 1 (LOXL1) is a lysyl oxidase, and emerging evidence has revealed its effect on malignant cancer progression. However, its role in colorectal cancer (CRC) and the underlying molecular mechanisms have not yet been elucidated.
Methods
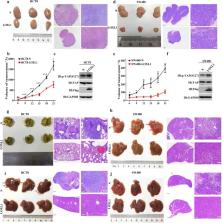
LOXL1 expression in colorectal cancer was detected by immunohistochemistry, western blotting and real-time PCR. In vitro, colony formation, wound healing, migration and invasion assays were performed to investigate the effects of LOXL1 on cell proliferation, migration and invasion. In vivo, metastasis models and mouse xenografts were used to assess tumorigenicity and metastasis ability. Molecular biology experiments were utilized to reveal the underlying mechanisms by which LOXL1 modulates the Hippo pathway.
Results
LOXL1 was highly expressed in normal colon tissues compared with cancer tissues. In vitro , silencing LOXL1 in CRC cell lines dramatically enhanced migration, invasion, and colony formation, while overexpression of LOXL1 exerted the opposite effects. The results of the in vivo experiments demonstrated that the overexpression of LOXL1 in CRC cell lines drastically inhibited metastatic progression and tumour growth. Mechanistically, LOXL1 inhibited the transcriptional activity of Yes-associated protein (YAP) by interacting with MST1/2 and increasing the phosphorylation of MST1/2.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm.
- Record: found
- Abstract: found
- Article: not found
Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance.
- Record: found
- Abstract: found
- Article: not found
