- Record: found
- Abstract: found
- Article: not found
Melanoma genome sequencing reveals frequent PREX2 mutations
Read this article at
Abstract
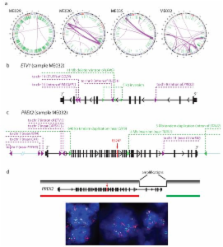
Melanoma is notable for its metastatic propensity, lethality in the advanced setting, and association with ultraviolet (UV) exposure early in life 1 . To obtain a comprehensive genomic view of melanoma, we sequenced the genomes of 25 metastatic melanomas and matched germline DNA. A wide range of point mutation rates was observed: lowest in melanomas whose primaries arose on non-UV exposed hairless skin of the extremities (3 and 14 per Mb genome), intermediate in those originating from hair-bearing skin of the trunk (range = 5 to 55 per Mb), and highest in a patient with a documented history of chronic sun exposure (111 per Mb). Analysis of whole-genome sequence data identified PREX2 - a PTEN-interacting protein and negative regulator of PTEN in breast cancer 2 - as a significantly mutated gene with a mutation frequency of approximately 14% in an independent extension cohort of 107 human melanomas. PREX2 mutations are biologically relevant, as ectopic expression of mutant PREX2 accelerated tumor formation of immortalized human melanocytes in vivo. Thus, whole-genome sequencing of human melanoma tumors revealed genomic evidence of UV pathogenesis and discovered a new recurrently mutated gene in melanoma.
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma.
- Record: found
- Abstract: found
- Article: not found
