- Record: found
- Abstract: found
- Article: found
Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application
Read this article at
Abstract
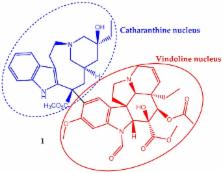
Cancer is a multistage process resulting in an uncontrolled and abrupt division of cells and is one of the leading causes of mortality. The cases reported and the predictions for the near future are unthinkable. Food and Drug Administration data showed that 40% of the approved molecules are natural compounds or inspired by them, from which, 74% are used in anticancer therapy. In fact, natural products are viewed as more biologically friendly, that is less toxic to normal cells. In this review, the most recent and successful cases of secondary metabolites, including alkaloid, diterpene, triterpene and polyphenolic type compounds, with great anticancer potential are discussed. Focusing on the ones that are in clinical trial development or already used in anticancer therapy, therefore successful cases such as paclitaxel and homoharringtonine (in clinical use), curcumin and ingenol mebutate (in clinical trials) will be addressed. Each compound’s natural source, the most important steps in their discovery, their therapeutic targets, as well as the main structural modifications that can improve anticancer properties will be discussed in order to show the role of plants as a source of effective and safe anticancer drugs.
Related collections
Most cited references127
- Record: found
- Abstract: found
- Article: not found
How Taxol/paclitaxel kills cancer cells
- Record: found
- Abstract: found
- Article: not found
Natural products: an evolving role in future drug discovery.
- Record: found
- Abstract: found
- Article: not found