- Record: found
- Abstract: found
- Article: found
Inflammation‐driven brain and gut barrier dysfunction in stress and mood disorders

Read this article at
Abstract
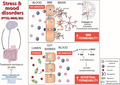
Regulation of emotions is generally associated exclusively with the brain. However, there is evidence that peripheral systems are also involved in mood, stress vulnerability vs. resilience, and emotion‐related memory encoding. Prevalence of stress and mood disorders such as major depression, bipolar disorder, and post‐traumatic stress disorder is increasing in our modern societies. Unfortunately, 30%–50% of individuals respond poorly to currently available treatments highlighting the need to further investigate emotion‐related biology to gain mechanistic insights that could lead to innovative therapies. Here, we provide an overview of inflammation‐related mechanisms involved in mood regulation and stress responses discovered using animal models. If clinical studies are available, we discuss translational value of these findings including limitations. Neuroimmune mechanisms of depression and maladaptive stress responses have been receiving increasing attention, and thus, the first part is centered on inflammation and dysregulation of brain and circulating cytokines in stress and mood disorders. Next, recent studies supporting a role for inflammation‐driven leakiness of the blood–brain and gut barriers in emotion regulation and mood are highlighted. Stress‐induced exacerbated inflammation fragilizes these barriers which become hyperpermeable through loss of integrity and altered biology. At the gut level, this could be associated with dysbiosis, an imbalance in microbial communities, and alteration of the gut–brain axis which is central to production of mood‐related neurotransmitter serotonin. Novel therapeutic approaches such as anti‐inflammatory drugs, the fast‐acting antidepressant ketamine, and probiotics could directly act on the mechanisms described here improving mood disorder‐associated symptomatology. Discovery of biomarkers has been a challenging quest in psychiatry, and we end by listing promising targets worth further investigation.
Abstract
Inflammation‐driven brain and gut barrier dysfunction in stress and mood disorders. Mood disorders, such as post‐traumatic stress disorder (PTSD), major depressive disorder (MDD), and bipolar disorder (BD), are associated with high rates of treatment resistance and relapse. Increasing evidence links blood–brain barrier (BBB) and gut barrier leakiness to negative emotional symptoms reported in mood disorders, possibly through stress‐induced inflammation, although specific biological mechanisms remain to be elucidated. Novel therapeutic approaches targeting BBB and gut barrier permeability could contribute to bridging the gap in treatment response.
Related collections
Most cited references501
- Record: found
- Abstract: found
- Article: not found
Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation
- Record: found
- Abstract: found
- Article: not found
The role of inflammation in depression: from evolutionary imperative to modern treatment target.
- Record: found
- Abstract: found
- Article: not found