- Record: found
- Abstract: found
- Article: found
Effects of chronic exposure to sodium arsenite on hypothalamo-pituitary-testicular activities in adult rats: possible an estrogenic mode of action
Read this article at
Abstract
Background
Inorganic arsenic is a major water pollutant and a known human carcinogen that has a suppressive influence on spermatogenesis and androgenesis in male reproductive system. However, the actual molecular events resulting in male reproductive dysfunctions from exposure to arsenic remain unclear. In this context, we evaluated the mode of action of chronic oral exposure of sodium arsenite on hypothalamo-pituitary- testicular activities in mature male albino rats.
Methods
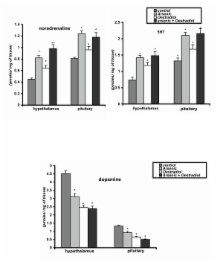
The effect of chronic oral exposure to sodium arsenite (5 mg/kg body weight/day) via drinking water without or with hCG (5 I.U./kg body weight/day) and oestradiol (25 micrograms oestradiol 3-benzoate suspended in 0.25 ml olive oil/rat/day) co-treatments for 6 days a week for 4 weeks (about the duration of two spermatogenic cycle) was evaluated in adult male rats. Changes in paired testicular weights, quantitative study of different varieties of germ cells at stage VII of spermatogenic cycle, epididymal sperm count, circulatory concentrations of hormones (LH, FSH, testosterone and corticosterone), testicular activities of delta 5, 3beta-hydroxysteroid dehydrogenase (delta 5, 3beta-HSD), 17 beta-hydroxysteroid dehydrogenase (17 beta-HSD), sorbitol dehydrogenase (SDH), acid phosphatase (ACP), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH), as well as the levels of biogenic amines (dopamine, noradrenaline and 5-hydroxytryptamine (5-HT)) in the hypothalamus and pituitary were monitored in this study. Hormones were assayed by radioimmuno- assay or enzyme- linked immunosorbent assay and the enzymes were estimated after spectrophotometry as well as the biogenic amines by HPLC electrochemistry.
Results
Sodium arsenite treatment resulted in: decreased paired testicular weights; epididymal sperm count; plasma LH, FSH, testosterone and testicular testosterone concentrations; and increased plasma concentration of corticosterone. Testicular enzymes such as delta 5, 3 beta-HSD, 17 beta-HSD, and sorbitol dehydrogenase (SDH) were significantly decreased, but those of acid phosphatase (ACP), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) were significantly increased. A decrease in dopamine or an increase in noradrenaline and 5-HT in hypothalamus and pituitary were also noted after arsenic exposure. Histological evaluation revealed extensive degeneration of different varieties of germ cells at stage VII of spermatogenic cycle in arsenic exposed rats. Administration of human chorionic gonadotrophin (hCG) along with sodium arsenite partially prevented the degeneration of germ cells and enhanced paired testicular weights, epididymal sperm count, plasma and intratesticular testosterone concentrations, activities of delta 5, 3beta-HSD, 17 beta-HSD and sorbitol dehydrogenase along with diminution in the activities of ACP, ALP and LDH. Since many of the observed arsenic effects could be enhanced by oestradiol, it is suggested that arsenic might somehow acts through an estrogenic mode of action.
Conclusion
The results indicate that arsenic causes testicular toxicity by germ cell degeneration and inhibits androgen production in adult male rats probably by affecting pituitary gonadotrophins. Estradiol treatment has been associated with similar effects on pituitary testicular axis supporting the hypothesis that arsenite might somehow act through an estrogenic mode of action.
Related collections
Most cited references86
- Record: found
- Abstract: found
- Article: not found
Estrogen and spermatogenesis.
- Record: found
- Abstract: found
- Article: not found
Transplacental carcinogenicity of inorganic arsenic in the drinking water: induction of hepatic, ovarian, pulmonary, and adrenal tumors in mice.
- Record: found
- Abstract: found
- Article: not found