- Record: found
- Abstract: found
- Article: found
Peripheral Dopamine in Restless Legs Syndrome
Read this article at
Abstract
Objective/Background
Restless Legs Syndrome (RLS) is a dopamine-dependent disorder characterized by a strong urge to move. The objective of this study was to evalulate blood levels of dopamine and other catecholamines and blood D2-subtype dopamine receptors (D2Rs) in RLS.
Patients/Methods
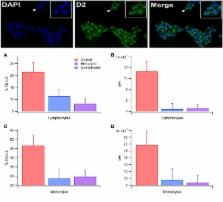
Dopamine levels in blood samples from age-matched unmedicated RLS subjects, medicated RLS subjects and Controls were evaluated with high performance liquid chromatography and dopamine D2R white blood cell (WBC) expression levels were determined with fluorescence-activated cell sorting and immunocytochemistry.
Results
Blood plasma dopamine levels, but not norepinepherine or epinephrine levels, were significantly increased in medicated RLS subjects vs unmedicated RLS subjects and Controls. The percentage of lymphocytes and monocytes expressing D2Rs differed between Control, RLS medicated and RLS unmedicated subjects. Total D2R expression in lymphocytes, but not monocytes, differed between Control, RLS medicated and RLS unmedicated subjects. D2Rs in lymphocytes, but not monocytes, were sensitive to dopamine in Controls only.
Conclusion
Downregulation of WBCs D2Rs occurs in RLS. This downregulation is not reversed by medication, although commonly used RLS medications increase plasma dopamine levels. The insensitivity of monocytes to dopamine levels, but their downregulation in RLS, may reflect their utility as a biomarker for RLS and perhaps brain dopamine homeostasis.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement.
- Record: found
- Abstract: found
- Article: not found
Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop.
- Record: found
- Abstract: found
- Article: not found