- Record: found
- Abstract: found
- Article: found
Synapse-specific opioid modulation of thalamo-cortico-striatal circuits
Read this article at
Abstract
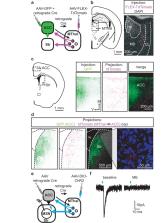
The medial thalamus (MThal), anterior cingulate cortex (ACC) and striatum play important roles in affective-motivational pain processing and reward learning. Opioids affect both pain and reward through uncharacterized modulation of this circuitry. This study examined opioid actions on glutamate transmission between these brain regions in mouse. Mu-opioid receptor (MOR) agonists potently inhibited MThal inputs without affecting ACC inputs to individual striatal medium spiny neurons (MSNs). MOR activation also inhibited MThal inputs to the pyramidal neurons in the ACC. In contrast, delta-opioid receptor (DOR) agonists disinhibited ACC pyramidal neuron responses to MThal inputs by suppressing local feed-forward GABA signaling from parvalbumin-positive interneurons. As a result, DOR activation in the ACC facilitated poly-synaptic (thalamo-cortico-striatal) excitation of MSNs by MThal inputs. These results suggest that opioid effects on pain and reward may be shaped by the relative selectivity of opioid drugs to the specific circuit components.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance.
- Record: found
- Abstract: found
- Article: not found
Functional imaging of brain responses to pain. A review and meta-analysis (2000).
- Record: found
- Abstract: not found
- Article: not found