- Record: found
- Abstract: found
- Article: not found
Melody, an ENU mutation in Caspase 3 , alters the catalytic cysteine residue and causes sensorineural hearing loss in mice
Read this article at
Abstract
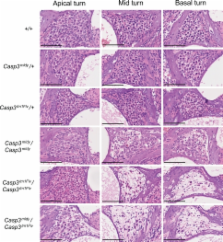
Progeny from the Harwell N-ethyl- N-nitrosourea (ENU) recessive mutagenesis screen were assessed for auditory defects. A pedigree was identified with multiple progeny lacking response to a clickbox test. Auditory brainstem response (ABR) analysis showed that homozygous mutant mice were profoundly deaf and the line was named melody. We subsequently mapped this mutation to a 6-Mb region on chromosome 8 and identified a point mutation in melody that results in a C163S substitution in the catalytic site of Caspase 3, a cysteine protease involved in apoptosis. Melody fails to complement a null Caspase-3 mutant. Scanning electron microscopy (SEM) has revealed disorganised sensory hair cells and hair cell loss. Histological analysis of melody has shown degeneration of spiral ganglion cells in homozygote mice, with a gradient of severity from apical to basal turns. Melody heterozygotes also show evidence of loss of spiral ganglion neurons, suggesting that the C163S mutation may show dominant negative effects by binding and sequestering proteins at the active site. The melody line provides a new model for studying the role of Caspase 3 in deafness and a number of other pathways and systems.
Related collections
Most cited references21
- Record: found
- Abstract: found
- Article: not found
Caspases: the executioners of apoptosis.
- Record: found
- Abstract: found
- Article: not found
Genome-wide, large-scale production of mutant mice by ENU mutagenesis.
- Record: found
- Abstract: found
- Article: not found
