- Record: found
- Abstract: found
- Article: found
Bupivacaine in alginate and chitosan nanoparticles: an in vivo evaluation of efficacy, pharmacokinetics, and local toxicity
Abstract
Objective
This study reports a preclinical evaluation of an alginate/chitosan nanoparticle formulation containing NovaBupi®, a racemic bupivacaine (BVC) containing 25% dextrobupivacaine and 75% levobupivacaine.
Methods
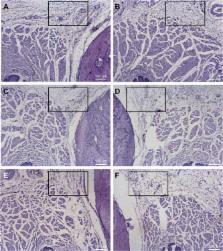
New Zealand White rabbits (n=6) received intraoral or intrathecal injections of BVC 0.5% or BVC 0.5%-loaded alginate–chitosan nanoparticles (BVC ALG). BVC plasma levels and pharmacokinetic parameters were determined in blood samples of these rabbits. An infraorbital nerve blockade was performed in male Wistar rats (n=7) with the same formulations and the vehicle (NP ALG). Histological evaluation of local toxicity after 6 hours and 24 hours of the treatments was performed in rats’ (n=6) oral tissues.
Results
No statistically significant difference was observed between plasma concentrations and pharmacokinetic parameters ( p>0.05) after intraoral injections. However, after intrathecal injection BVC ALG changed approximately three times the values of volume of distribution and area under the curve (AUC 0–t; p<0.05). The total analgesic effect of BVC after infraorbital nerve blockade was improved by 1.4-fold ( p<0.001) with BVC ALG. BVC and BVC ALG did not induce significant local inflammatory reaction.
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Anti-inflammatory properties of local anesthetics and their present and potential clinical implications.
- Record: found
- Abstract: found
- Article: not found
Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine.
- Record: found
- Abstract: found
- Article: not found