- Record: found
- Abstract: found
- Article: found
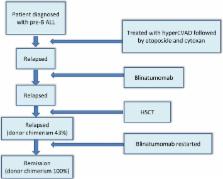
Blinatumomab may induce graft versus host leukemia in patients with pre‐B ALL relapsing after hematopoietic stem cell transplant
case-report
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Key Clinical Message
Blinatumomab, a bispecific T‐cell engager monoclonal antibody used to manage Philadelphia chromosome‐negative relapsed or refractory B‐cell precursor acute lymphoblastic leukemia ( ALL) can be used to treat patients by inducing graft versus leukemia reaction post allogeneic hematopoietic stem cell transplantation, a feature which it was post allogeneic bone marrow transplantation, a feature which this drug was not aimed to do.
Related collections
Most cited references2
- Record: found
- Abstract: found
- Article: not found
Reporting results of cancer treatment.
A Miller, B Hoogstraten, M. Staquet … (1981)
- Record: found
- Abstract: found
- Article: not found
Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival.
Max Topp, Peter Kufer, Nicola Gökbuget … (2011)