- Record: found
- Abstract: found
- Article: not found
Solution Catalytic Cycle of Incompatible Steps for Ambient Air Oxidation of Methane to Methanol

Read this article at
Abstract
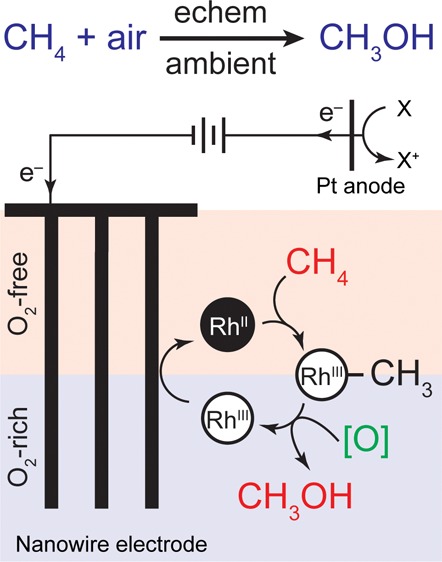
Direct chemical synthesis from methane and air under ambient conditions is attractive yet challenging. Low-valent organometallic compounds are known to activate methane, but their electron-rich nature seems incompatible with O 2 and prevents catalytic air oxidation. We report selective oxidation of methane to methanol with an O 2-sensitive metalloradical as the catalyst and air as the oxidant at room temperature and ambient pressure. The incompatibility between C–H activation and O 2 oxidation is reconciled by electrochemistry and nanomaterials, with which a concentration gradient of O 2 within the nanowire array spatially segregated incompatible steps in the catalytic cycle. An unexpected 220 000-fold increase of the apparent reaction rate constants within the nanowire array leads to a turnover number up to 52 000 within 24 h. The synergy between nanomaterials and organometallic chemistry warrants a new catalytic route for CH 4 functionalization.
Abstract
Nanowire array promotes the separation of incompatible reaction steps to allow air-sensitive molecules to catalytically oxidize natural gas to alcohols under ambient conditions.
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Metal-assisted chemical etching of silicon: a review.
- Record: found
- Abstract: found
- Article: not found
Platinum catalysts for the high-yield oxidation of methane to a methanol derivative
- Record: found
- Abstract: found
- Article: not found
