- Record: found
- Abstract: found
- Article: found
Emerging Role of One-Carbon Metabolism and DNA Methylation Enrichment on δ-Containing GABA A Receptor Expression in the Cerebellum of Subjects with Alcohol Use Disorders (AUD)
Read this article at
Abstract
Background
Cerebellum is an area of the brain particularly sensitive to the effects of acute and chronic alcohol consumption. Alcohol exposure decreases cerebellar Purkinje cell output by increasing GABA release from Golgi cells onto extrasynaptic α 6/δ-containing GABA A receptors located on glutamatergic granule cells. Here, we studied whether chronic alcohol consumption induces changes in GABA A receptor subunit expression and whether these changes are associated with alterations in epigenetic mechanisms via DNA methylation.
Methods
We used a cohort of postmortem cerebellum from control and chronic alcoholics, here defined as alcohol use disorders subjects (n=25/group). S-adenosyl-methionine/ S-adenosyl-homocysteine were measured by high-performance liquid chromatography. mRNA levels of various genes were assessed by reverse transcriptase-quantitative polymerase chain reaction. Promoter methylation enrichment was assessed using methylated DNA immunoprecipitation and hydroxy-methylated DNA immunoprecipitation assays.
Results
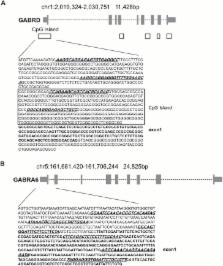
mRNAs encoding key enzymes of 1-carbon metabolism that determine the S-adenosyl-methionine/ S-adenosyl-homocysteine ratio were increased, indicating higher “methylation index” in alcohol use disorder subjects. We found that increased methylation of the promoter of the δ subunit GABA A receptor was associated with reduced mRNA and protein levels in the cerebellum of alcohol use disorder subjects. No changes were observed in α 1- or α 6-containing GABA A receptor subunits. The expression of DNA-methyltransferases (1, 3A, and 3B) was unaltered, whereas the mRNA level of TET1, which participates in the DNA demethylation pathway, was decreased. Hence, increased methylation of the δ subunit GABA A receptor promoter may result from alcohol-induced reduction of DNA demethylation.
Conclusion
Together, these results support the hypothesis that aberrant DNA methylation pathways may be involved in cerebellar pathophysiology of alcoholism. Furthermore, this work provides novel evidence for a central role of DNA methylation mechanisms in the alcohol-induced neuroadaptive changes of human cerebellar GABA A receptor function.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Hydroxylation of 5-methylcytosine by TET1 promotes active DNA demethylation in the adult brain.
- Record: found
- Abstract: found
- Article: not found
Control of mental activities by internal models in the cerebellum.
- Record: found
- Abstract: found
- Article: not found