- Record: found
- Abstract: found
- Article: found
A comparison between the administration of oral prolonged-release oxycodone-naloxone and transdermal fentanyl in patients with moderate-to-severe cancer pain: a propensity score analysis
Abstract
Background
Opioids are the most important pharmacological treatment for moderate-to-severe cancer pain, but side effects limit their use. Transdermal fentanyl (TDF) and oral prolonged-release oxycodone-naloxone (OXN-PR) are effective in controlling chronic pain, with less constipation compared to other opioids. However, TDF and OXN-PR have never been directly compared.
Patients and methods
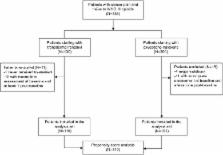
Cancer patients with moderate-to-severe chronic pain were consecutively enrolled in two prospective 28-day trials, received either TDF or OXN-PR, and were assessed at baseline and after 7, 14, 21, and 28 days. The primary endpoint was 28-day analgesic response rate (average pain intensity decrease ≥30% from baseline). Other outcome measures included opioid daily dose changes over time; need for adjuvant analgesics; number of switches; premature discontinuation; presence and severity of constipation; and other adverse drug reactions. To compare the efficacy and the safety of TDF and OXN-PR, we used the propensity score analysis to adjust for heterogeneity between the two patient groups.
Results
Three hundred ten out of 336 patients originally treated (119 TDF and 191 OXN-PR) were included in the comparative analysis. The amount of responders was comparable after TDF (75.3%) and OXN-PR administration (82.9%, not significant [NS]). The final opioid daily dose expressed as morphine equivalent was 113.6 mg for TDF and 44.5 mg for OXN-PR ( p<0.0001). A daily opioid dose escalation >5% was less common after OXN-PR (19.3%) than after TDS administration (37.9%, p<0.0001). Opioid switches and discontinuation were similar in both groups. Severe constipation in the two groups was comparable (32.6% after TDF vs 24.7% after OXN-PR, NS). Nausea, vomiting, and dry mouth were significantly less frequent in the OXN-PR group than in the TDF group.
Most cited references27
- Record: found
- Abstract: found
- Article: not found
Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC.
- Record: found
- Abstract: not found
- Article: not found
Management of cancer pain: ESMO Clinical Practice Guidelines.
- Record: found
- Abstract: found
- Article: not found