- Record: found
- Abstract: found
- Article: found
Tumor microenvironment in gastric cancers

Read this article at
Abstract
The tumor microenvironment favors the growth and expansion of cancer cells. Many cell types are involved in the tumor microenvironment such as inflammatory cells, fibroblasts, nerves, and vascular endothelial cells. These stromal cells contribute to tumor growth by releasing various molecules to either directly activate the growth signaling in cancer cells or remodel surrounding areas. This review introduces recent advances in findings on the interactions within the tumor microenvironment such as in cancer‐associated fibroblasts (CAFs), immune cells, and endothelial cells, in particular those established in mouse gastric cancer models. In mice, myofibroblasts in the gastric stroma secrete R‐spondin and support normal gastric stem cells. Most CAFs promote tumor growth in a paracrine manner, but CAF population appears to be heterogeneous in terms of their function and origin, and include both tumor‐promoting and tumor‐restraining populations. Among immune cell populations, tumor‐associated macrophages, including M1 and M2 macrophages, and myeloid‐derived suppressor cells (MDSCs), are reported to directly or indirectly promote gastric tumorigenesis by secreting soluble factors or modulating immune responses. Endothelial cells or blood vessels not only fuel tumors with nutrients, but also interact with cancer stem cells and immune cells by secreting chemokines or cytokines, and act as a cancer niche. Understanding these interactions within the tumor microenvironment would contribute to unraveling new therapeutic targets.
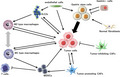
Abstract
Gastric tumor microenvironment: Cancer‐associated fibroblasts, endothelial cells, gastrin‐expressing cells, and various immune cells including macrophages, MDSCs, and ILC2s serve as tumor‐promoting niche in gastric cancers. There are numerous crosstalks between tumor cells and surrounding stromal cell types, which contribute to tumor development derived from gastric stem cells.
Related collections
Most cited references74
- Record: found
- Abstract: found
- Article: not found
Origins of tumor-associated macrophages and neutrophils.
- Record: found
- Abstract: found
- Article: not found
Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1.

- Record: found
- Abstract: found
- Article: found