- Record: found
- Abstract: found
- Article: found
A comprehensive proteomics profiling identifies NRP1 as a novel identity marker of human bone marrow mesenchymal stromal cell-derived small extracellular vesicles
Read this article at
Abstract
Background
Clinical applications have shown extracellular vesicles (EVs) to be a major paracrine effector in therapeutic responses produced by human mesenchymal stromal/stem cells (hMSCs). As the regenerative capacity of EVs is mainly ascribed to the transfer of proteins and RNA composing its cargo, and to the activity attributed by the protein surface markers, we sought to profile the protein composition of small EVs released from hMSCs to identify hMSC-EV biomarkers with potential clinical relevance.
Methods
Small EVs were produced and qualified from five human bone marrow MSC donors at low passage following a 48-h culture in exosome-depleted medium further processed by steps of centrifugation, filtration, and precipitation. Quantitative proteomic analysis comparing the protein profile of the EVs released from hMSCs and their parental cell was conducted using tandem mass tag labeling combined to mass spectrometry (LC-MS/MS) to identify enriched EV protein markers.
Results
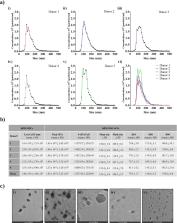
Nanoparticle tracking analysis showed no differences in the EV concentration and size among the five hMSC donors (1.83 × 10 10 ± 3.23 × 10 9/mL), with the mode particle size measuring at 109.3 ± 5.7 nm. Transmission electron microscopy confirmed the presence of nanovesicles with bilayer membranes. Flow cytometric analysis identified commonly found exosomal (CD63/CD81) and hMSC (CD105/CD44/CD146) markers from released EVs in addition to surface mediators of migration (CD29 and MCSP). Quantitative proteomic identified 270 proteins significantly enriched by at least twofold in EVs released from hMSCs as compared to parental hMSCs, where neuropilin 1 (NRP1) was identified among 21 membrane-bound proteins regulating the migration and invasion of cells, as well as chemotaxis and vasculogenesis. Validation by western blot of multiple batches of EVs confirmed consistent enrichment of NRP1 in the nanovesicles released from all five hMSC donors.
Related collections
Most cited references27
- Record: found
- Abstract: found
- Article: found
Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications
- Record: found
- Abstract: found
- Article: not found
Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease.
- Record: found
- Abstract: found
- Article: not found