- Record: found
- Abstract: found
- Article: found
Adsorption of Heavy Metals by Graphene Oxide/Cellulose Hydrogel Prepared from NaOH/Urea Aqueous Solution
Read this article at
Abstract
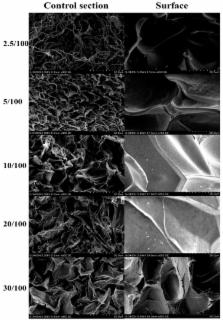
By taking advantage of cellulose, graphene oxide (GO), and the process for crosslinking using epichlorohydrin (ECH), we propose a simple and novel method to prepare GO/cellulose hydrogel with good potential to adsorb metal ions. GO nanosheets containing carboxyl and hydroxyl groups were introduced into the surface of the cellulose hydrogel with retention of the gel structure and its nanoporous property. Due to the introduction of GO, the GO/cellulose composite hydrogels exhibited good compressive strength. Adsorption capacity of Cu 2+ significantly increases with an increase in the GO/cellulose ratio and GO/cellulose hydrogel showed high adsorption rates. The calculated adsorption capacities at equilibrium ( ) for GO/cellulose hydrogel (GO:cellulose = 20:100 in weight) was up to 94.34 mg·g −1, which was much higher than that of the pristine cellulose hydrogels. Furthermore, GO/cellulose hydrogel exhibited high efficient regeneration and metal ion recovery, and high adsorption capacity for Zn 2+, Fe 3+, and Pb 2+.
Related collections
Most cited references41
- Record: found
- Abstract: not found
- Article: not found
Cellulose-based hydrogels: Present status and application prospects
- Record: found
- Abstract: found
- Article: not found
Adsorption of basic dye on high-surface-area activated carbon prepared from coconut husk: equilibrium, kinetic and thermodynamic studies.
- Record: found
- Abstract: found
- Article: not found