- Record: found
- Abstract: found
- Article: found
Reduction of voltage gated sodium channel protein in DRG by vector mediated miRNA reduces pain in rats with painful diabetic neuropathy
Read this article at
Abstract
Background
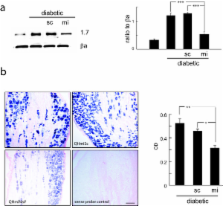
Painful neuropathy is a common complication of diabetes. Previous studies have identified significant increases in the amount of voltage gated sodium channel isoforms Na V1.7 and Na V1.3 protein in the dorsal root ganglia (DRG) of rats with streptozotocin (STZ)-induced diabetes. We found that gene transfer-mediated release of the inhibitory neurotransmitters enkephalin or gamma amino butyric acid (GABA) from DRG neurons in diabetic animals reduced pain-related behaviors coincident with a reduction in Na V1.7 protein levels in DRG in vivo. To further evaluate the role of Na Vα subunit levels in DRG in the pathogenesis of pain in diabetic neuropathy, we constructed a non-replicating herpes simplex virus (HSV)-based vector expressing a microRNA (miRNA) against Na Vα subunits.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia.
- Record: found
- Abstract: found
- Article: not found
The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways.
- Record: found
- Abstract: found
- Article: not found