- Record: found
- Abstract: found
- Article: found
Feasibility of Non-TBI Conditioning with Busulfan and Fludarabine for Allogeneic Stem Cell Transplantation in Lymphoid Malignancy
Read this article at
Abstract
Background/Aims
This retrospective study evaluated the transplantation outcomes of patients with adult lymphoid malignancies who received chemotherapy-based conditioning with busulfan and fludarabine (BuFlu) and busulfan and cyclophosphamide (BuCy2).
Methods
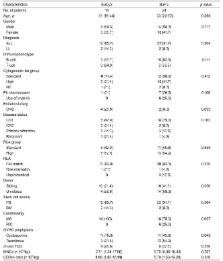
Thirty-eight patients (34 with acute lymphoblastic leukemia and 4 with lymphoblastic lymphoma) were included in the current study. The conditioning regimen was BuCy2 for 14 patients and BuFlu for the remaining 24 patients. Eight and 13 patients were high risk disease in the BuCy2 and BuFlu groups, respectively.
Results
The cumulative incidence of grade II-IV acute graft-versus-host disease (GVHD) was 56.5% and 55.2% and that of extensive chronic GVHD 17.0% and 55.6% (p = 0.018) for the BuFlu and BuCy2 groups, respectively. The 3-year relapse rate was 27.8% and 31.4% and 3-year overall survival 34.3% and 46.8% for the BuFlu and BuCy2 groups, respectively. Treatment-related mortality (TRM) was significantly lower in the BuFlu group (16.9%) than in the BuCy2 group (57.1%, p = 0.010). In multivariate analyses, the BuFlu regimen was identified as an independent favorable risk factor for TRM (hazard ratio [HR], 0.036; p = 0.017) and extensive chronic GVHD (HR, 0.168; p = 0.034).
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993).
- Record: found
- Abstract: found
- Article: not found
Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients.
- Record: found
- Abstract: found
- Article: not found