- Record: found
- Abstract: found
- Article: found
PICK1 inhibits the E3 ubiquitin ligase activity of Parkin and reduces its neuronal protective effect
Read this article at
Significance
Parkinson’s disease (PD) is the second most common neurodegenerative disorder. It is characterized by progressive deterioration of motor function caused by loss of dopamine-producing neurons. Currently, there is no treatment that could stop the progress of the disease. Loss-of-function mutations in Parkin account for ∼50% of early onset PD. Parkin functions as an E3 ubiquitin ligase that exhibits multiple protective roles especially in dopaminergic neurons. Here, we demonstrate that PICK1 directly binds to Parkin. PICK1 is a potent endogenous inhibitor of Parkin’s E3 ubiquitin ligase activity and blocks Parkin’s protective functions. Conversely, knockout of PICK1 enhances the neuroprotective effect of Parkin. Thus, the reduction of PICK1 may provide a therapeutic target for the treatment of PD.
Abstract
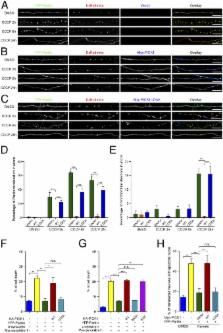
Parkin functions as a multipurpose E3 ubiquitin ligase, and Parkin loss of function is associated with both sporadic and familial Parkinson’s disease (PD). We report that the Bin/Amphiphysin/Rvs (BAR) domain of protein interacting with PRKCA1 (PICK1) bound to the really interesting new gene 1 (RING1) domain of Parkin and potently inhibited the E3 ligase activity of Parkin by disrupting its interaction with UbcH7. Parkin translocated to damaged mitochondria and led to their degradation in neurons, whereas PICK1 robustly inhibited this process. PICK1 also impaired the protective function of Parkin against stresses in SH-SY5Y cells and neurons. The protein levels of several Parkin substrates were reduced in young PICK1-knockout mice, and these mice were resistant to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-mediated toxicity. Taken together, the results indicate that PICK1 is a potent inhibitor of Parkin, and the reduction of PICK1 enhances the protective effect of Parkin.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Association between early-onset Parkinson's disease and mutations in the parkin gene.
- Record: found
- Abstract: found
- Article: not found
Selective autophagy: ubiquitin-mediated recognition and beyond.
- Record: found
- Abstract: found
- Article: not found