- Record: found
- Abstract: found
- Article: found
“Let’s get the best quality research we can”: public awareness and acceptance of consent to use existing data in health research: a systematic review and qualitative study
Read this article at
Abstract
Background
Opt-in consent is usually required for research, but is known to introduce selection bias. This is a particular problem for large scale epidemiological studies using only pre-collected health data. Most previous studies have shown that members of the public value opt-in consent and can perceive research without consent as an invasion of privacy. Past research has suggested that people are generally unaware of research processes and existing safeguards, and that education may increase the acceptability of research without prior informed consent, but this recommendation has not been formally evaluated. Our objectives were to determine the range of public opinion about the use of existing medical data for research and to explore views about consent to a secondary review of medical records for research. We also investigated the effect of the provision of detailed information about the potential effect of selection bias on public acceptability of the use of data for research.
Methods
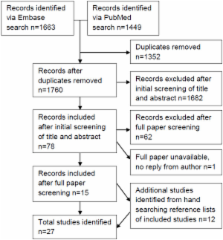
We carried out a systematic review of existing literature on public attitudes to secondary use of existing health records identified by searching PubMed (1966-present), Embase (1974-present) and reference lists of identified studies to provide a general overview, followed by a qualitative focus group study with 19 older men recruited from rural and suburban primary care practices in the UK to explore key issues in detail.
Results
The systematic review identified twenty-seven relevant papers and the findings suggested that males and older people were more likely to consent to a review of their medical data. Many studies noted participants’ lack of knowledge about research processes and existing safeguards and this was reflected in the focus groups. Focus group participants became more accepting of the use of pre-collected medical data without consent after being given information about selection bias and research processes. All participants were keen to contribute to NHS-related research but some were concerned about data-sharing for commercial gain and the potential misuse of information.
Conclusions
Increasing public education about research and specific targeted information provision could promote trust in research processes and safeguards, which in turn could increase the acceptability of research without specific consent where the need for consent would lead to biased findings and impede research necessary to improve public health.
Related collections
Most cited references34

- Record: found
- Abstract: found
- Article: found
Written informed consent and selection bias in observational studies using medical records: systematic review
- Record: found
- Abstract: found
- Article: not found
Patterns of consent in epidemiologic research: evidence from over 25,000 responders.
- Record: found
- Abstract: found
- Article: not found