- Record: found
- Abstract: found
- Article: found
Successful tumour necrosis factor (TNF) blocking therapy suppresses oxidative stress and hypoxia-induced mitochondrial mutagenesis in inflammatory arthritis
Read this article at
Abstract
Introduction
To examine the effects of tumour necrosis factor (TNF) blocking therapy on the levels of early mitochondrial genome alterations and oxidative stress.
Methods
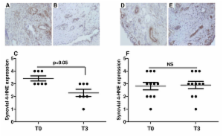
Eighteen inflammatory arthritis patients underwent synovial tissue oxygen (tpO 2) measurements and clinical assessment of disease activity (DAS28-CRP) at baseline (T0) and three months (T3) after starting biologic therapy. Synovial tissue lipid peroxidation (4-HNE), T and B cell specific markers and synovial vascular endothelial growth factor (VEGF) were quantified by immunohistochemistry. Synovial levels of random mitochondrial DNA (mtDNA) mutations were assessed using Random Mutation Capture (RMC) assay.
Results
4-HNE levels pre/post anti TNF-α therapy were inversely correlated with in vivo tpO 2 ( P < 0.008; r = -0.60). Biologic therapy responders showed a significantly reduced 4-HNE expression ( P < 0.05). High 4-HNE expression correlated with high DAS28-CRP ( P = 0.02; r = 0.53), tender joint count for 28 joints (TJC-28) ( P = 0.03; r = 0.49), swollen joint count for 28 joints (SJC-28) ( P = 0.03; r = 0.50) and visual analogue scale (VAS) ( P = 0.04; r = 0.48). Strong positive association was found between the number of 4-HNE positive cells and CD4+ cells ( P = 0.04; r = 0.60), CD8+ cells ( P = 0.001; r = 0.70), CD20+ cells ( P = 0.04; r = 0.68), CD68+ cells ( P = 0.04; r = 0.47) and synovial VEGF expression ( P = 0.01; r = 063). In patients whose in vivo tpO 2 levels improved post treatment, significant reduction in mtDNA mutations and DAS28-CRP was observed ( P < 0.05). In contrast in those patients whose tpO 2 levels remained the same or reduced at T3, no significant changes for mtDNA mutations and DAS28-CRP were found.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis.
- Record: found
- Abstract: found
- Article: not found
The role of reactive oxygen species in homeostasis and degradation of cartilage.
- Record: found
- Abstract: found
- Article: not found