- Record: found
- Abstract: found
- Article: found
A randomized, double-blind clinical trial of canrenone vs hydrochlorothiazide in addition to angiotensin II receptor blockers in hypertensive type 2 diabetic patients
Abstract
Aim
The aim of this study was to evaluate the effects of canrenone compared to hydrochlorothiazide (HCTZ) added to angiotensin II receptor blockers (ARBs) on glycemia, lipid profile, potassium, aldosterone and renal function in patients with hypertension and type 2 diabetes mellitus.
Patients and methods
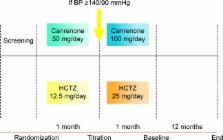
The study enrolled 182 Caucasian patients with hypertension and type 2 diabetes mellitus. Patients were already taking ARBs and were randomized to canrenone, 50 mg once a day, or HCTZ, 12.5 mg once a day for 1 month. After the first month, patients not reaching an adequate blood pressure (BP) were up-titrated to canrenone 100 mg or HCTZ 25 mg once a day for 12 months. The following parameters were considered at 6 and 12 months: BP, body weight, body mass index (BMI), fasting plasma glucose (FPG), post-prandial glucose (PPG), fasting plasma insulin (FPI), homeostasis model assessment insulin (HOMA-IR), lipid profile, potassium, plasma aldosterone, urine albumin excretion rate and estimated glomerular filtration rate (eGFR).
Results
We observed a similar decrease in BP with both treatments. Canrenone led to a significant decrease in FPG, PPG and HOMA index compared to baseline, while there was a significant increase in the same parameters with HCTZ. HCTZ also worsened glycated hemoglobin (HbA 1c), while canrenone did not change it. No variations in lipid profile were recorded with canrenone, while there was a worsening of total cholesterol (TC) and triglycerides (Tg) with HCTZ. Potassium levels were decreased and uric acid levels were increased by HCTZ, but not by canrenone that had a neutral effect on these parameters. We recorded a slight decrease in eGFR with HCTZ and an improvement with canrenone; creatinine and eGFR were improved by canrenone compared to HCTZ. Plasma aldosterone levels were decreased by canrenone and increased by HCTZ.
Most cited references14
- Record: found
- Abstract: not found
- Article: not found
Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD).
- Record: found
- Abstract: not found
- Article: not found
Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006.
- Record: found
- Abstract: found
- Article: not found