- Record: found
- Abstract: found
- Article: found
Maturation of Corticospinal Tracts in Children With Hemiplegic Cerebral Palsy Assessed by Diffusion Tensor Imaging and Transcranial Magnetic Stimulation
Read this article at
Abstract
Aim: To assess changes in the developmental trajectory of corticospinal tracts (CST) maturation in children with hemiplegic cerebral palsy (HCP).
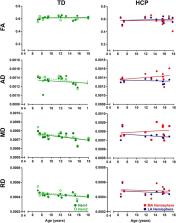
Methods: Neuroimaging data were obtained from 36 children with HCP for both the more affected (MA) and less affected (LA) hemispheres, and, for purposes of direct comparison, between groups, 15 typically developing (TD) children. With diffusion tensor imaging (DTI), we estimated the mean fractional anisotropy (FA), axial diffusivity (AD), mean diffusivity (MD), and radial diffusivity (RD) of the corticospinal tract, parameters indicative of factors including myelination and axon density. Transcranial magnetic stimulation (TMS) was performed as a neurophysiologic measure of corticospinal tract integrity and organization. Resting motor threshold (rMT) was obtained per hemisphere, per patient.
Results: We observed a significant AD and MD developmental trajectory, both of which were inversely related to age (decrease in AD and diffusivity corresponding to increased age) in both hemispheres of TD children ( p < 0.001). This maturation process was absent in both MA and LA hemispheres of children with HCP. Additionally, the TMS-derived previously established rMT developmental trajectory was preserved in the LA hemisphere of children with HCP ( n = 26; p < 0.0001) but this trajectory was absent in the MA hemisphere.
Conclusions: Corticospinal tract maturation arrests in both hemispheres of children with HCP, possibly reflecting perinatal disruption of corticospinal tract myelination and axonal integrity.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
A report: the definition and classification of cerebral palsy April 2006.
- Record: found
- Abstract: found
- Article: not found
Demyelination increases radial diffusivity in corpus callosum of mouse brain.
- Record: found
- Abstract: found
- Article: not found