- Record: found
- Abstract: found
- Article: found
Oral formulation of DPP-4 inhibitor plus Quercetin improves metabolic homeostasis in type 1 diabetic rats
Read this article at
Abstract
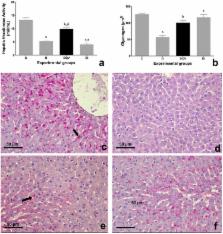
This study aimed to investigate the potential of an oral formulation (QV formulation) containing Quercetin and a Dipeptidyl Peptidase-4 Inhibitor (DPP-4 inhibitor), Vildagliptin, in improving metabolic homeostasis in type 1 diabetes model. Female albino Fischer rats were divided into four groups: untreated control animals (C), untreated diabetic animals (D), diabetic animals treated with QV formulation (DQV), and diabetic animals treated with insulin (DI). Diabetes was induced by injection of alloxan (135 mg kg body mass) −1 and confirmed by glycemic test. After the 30-day treatment period, biochemical parameters were analyzed in the pancreas, liver, and serum. Histopathological changes in pancreatic tissue were examined by Hematoxyline & Eosin staining and the insulin content in the islet measured by immunohistochemistry with anti-insulin antibody. The glycogen content in the hepatocytes was quantified by Periodic Schiff Acid staining. The QV formulation reduced the glycemia, preserved the pancreatic architecture, increased insulin levels, furthermore ameliorated lipid profile and to promote higher survival rate of animals. Together, our data suggest that the QV formulation treatment was able to normalize metabolic homeostasis in type 1 diabetic rats.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Microneedle-array patches loaded with hypoxia-sensitive vesicles provide fast glucose-responsive insulin delivery.
- Record: found
- Abstract: found
- Article: not found
