- Record: found
- Abstract: found
- Article: found
Involvement of the Nucleus Incertus and Relaxin-3/RXFP3 Signaling System in Explicit and Implicit Memory
Read this article at
Abstract
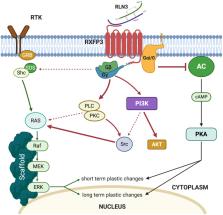
Telencephalic cognitive and emotional circuits/functions are strongly modulated by subcortical inputs. The main focus of past research on the nature of this modulation has been on the widespread monoamine projections to the telencephalon. However, the nucleus incertus (NI) of the pontine tegmentum provides a strong GABAergic and peptidergic innervation of the hippocampus, basal forebrain, amygdala, prefrontal cortex, and related regions; and represents a parallel source of ascending modulation of cognitive and emotional domains. NI GABAergic neurons express multiple peptides, including neuromedin-B, cholecystokinin, and relaxin-3, and receptors for stress and arousal transmitters, including corticotrophin-releasing factor and orexins/hypocretins. A functional relationship exists between NI neurons and their associated peptides, relaxin-3 and neuromedin-B, and hippocampal theta rhythm, which in turn, has a key role in the acquisition and extinction of declarative and emotional memories. Furthermore, RXFP3, the cognate receptor for relaxin-3, is a G i/o protein-coupled receptor, and its activation inhibits the cellular accumulation of cAMP and induces phosphorylation of ERK, processes associated with memory formation in the hippocampus and amygdala. Therefore, this review summarizes the role of NI transmitter systems in relaying stress- and arousal-related signals to the higher neural circuits and processes associated with memory formation and retrieval.
Related collections
Most cited references133
- Record: found
- Abstract: found
- Article: not found
Memory, navigation and theta rhythm in the hippocampal-entorhinal system.
- Record: found
- Abstract: not found
- Article: not found
The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat
- Record: found
- Abstract: found
- Article: not found