- Record: found
- Abstract: found
- Article: found
Altered metabolic landscape in IDH‐mutant gliomas affects phospholipid, energy, and oxidative stress pathways
Read this article at
Abstract
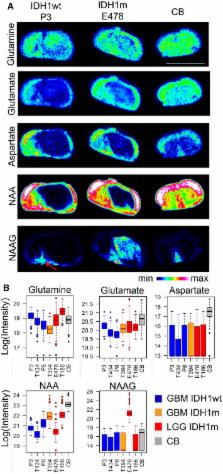
Heterozygous mutations in NADP‐dependent isocitrate dehydrogenases ( IDH) define the large majority of diffuse gliomas and are associated with hypermethylation of DNA and chromatin. The metabolic dysregulations imposed by these mutations, whether dependent or not on the oncometabolite D‐2‐hydroxyglutarate (D2 HG), are less well understood. Here, we applied mass spectrometry imaging on intracranial patient‐derived xenografts of IDH‐mutant versus IDH wild‐type glioma to profile the distribution of metabolites at high anatomical resolution in situ. This approach was complemented by in vivo tracing of labeled nutrients followed by liquid chromatography–mass spectrometry ( LC‐ MS) analysis. Selected metabolites were verified on clinical specimen. Our data identify remarkable differences in the phospholipid composition of gliomas harboring the IDH1 mutation. Moreover, we show that these tumors are characterized by reduced glucose turnover and a lower energy potential, correlating with their reduced aggressivity. Despite these differences, our data also show that D2 HG overproduction does not result in a global aberration of the central carbon metabolism, indicating strong adaptive mechanisms at hand. Intriguingly, D2 HG shows no quantitatively important glucose‐derived label in IDH‐mutant tumors, which suggests that the synthesis of this oncometabolite may rely on alternative carbon sources. Despite a reduction in NADPH, glutathione levels are maintained. We found that genes coding for key enzymes in de novo glutathione synthesis are highly expressed in IDH‐mutant gliomas and the expression of cystathionine‐β‐synthase ( CBS ) correlates with patient survival in the oligodendroglial subtype. This study provides a detailed and clinically relevant insight into the in vivo metabolism of IDH1‐mutant gliomas and points to novel metabolic vulnerabilities in these tumors.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal.
- Record: found
- Abstract: found
- Article: not found
Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas.
- Record: found
- Abstract: found
- Article: not found