- Record: found
- Abstract: found
- Article: found
PAR1 Scaffolds TGFβRII to Downregulate TGF-β Signaling and Activate ESC Differentiation to Endothelial Cells

Read this article at
Summary
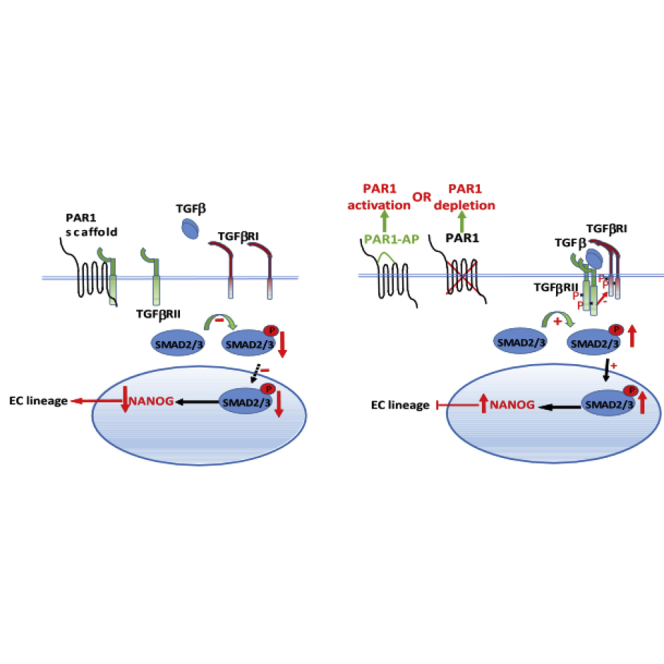
We studied the function of the G-protein-coupled receptor PAR1 in mediating the differentiation of mouse embryonic stem cells (mESCs) to endothelial cells (ECs) that are capable of inducing neovascularization. We observed that either deletion or activation of PAR1 suppressed mouse embryonic stem cell (mESC) differentiation to ECs and neovascularization in mice. This was mediated by induction of TGFβRII/TGFβRI interaction, forming an active complex, which in turn induced SMAD2 phosphorylation. Inhibition of TGF-β signaling in PAR1-deficient mESCs restored the EC differentiation potential of mESCs. Thus, PAR1 in its inactive unligated state functions as a scaffold for TGFβRII to downregulate TGF-β signaling, and thereby promote ESC transition to functional ECs. The PAR1 scaffold function in ESCs is an essential mechanism for dampening TGF-β signaling and regulating ESC differentiation.
Graphical Abstract
Highlights
Abstract
In this article, Malik and colleagues show that the G-protein-coupled receptor PAR1 can act as a scaffold for the TGF-β receptor TGFβRII and thereby suppress downstream TGF-β signaling in embryonic stem cells undergoing endothelial differentiation. Thus, PAR1 functions as a rheostat controlling TGF-β signaling and the generation of functional endothelial cells from embryonic stem cells.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Thrombin signalling and protease-activated receptors.
- Record: found
- Abstract: found
- Article: not found