- Record: found
- Abstract: found
- Article: found
Targeting malaria parasite invasion of red blood cells as an antimalarial strategy
Read this article at
Abstract
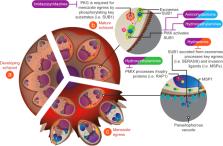
Plasmodium spp. parasites that cause malaria disease remain a significant global-health burden. With the spread of parasites resistant to artemisinin combination therapies in Southeast Asia, there is a growing need to develop new antimalarials with novel targets. Invasion of the red blood cell by Plasmodium merozoites is essential for parasite survival and proliferation, thus representing an attractive target for therapeutic development. Red blood cell invasion requires a co-ordinated series of protein/protein interactions, protease cleavage events, intracellular signals, organelle release and engagement of an actin-myosin motor, which provide many potential targets for drug development. As these steps occur in the bloodstream, they are directly susceptible and exposed to drugs. A number of invasion inhibitors against a diverse range of parasite proteins involved in these different processes of invasion have been identified, with several showing potential to be optimised for improved drug-like properties. In this review, we discuss red blood cell invasion as a drug target and highlight a number of approaches for developing antimalarials with invasion inhibitory activity to use in future combination therapies.
Abstract
Malaria invasion of red blood cells is an essential step in parasite replication and this review discusses targets and drug chemotypes being developed to stop invasion and growth.
Related collections
Most cited references157
- Record: found
- Abstract: found
- Article: not found
Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection.
- Record: found
- Abstract: found
- Article: not found
Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial.
- Record: found
- Abstract: found
- Article: not found