- Record: found
- Abstract: found
- Article: found
Evaluation of Macitentan in Patients With Eisenmenger Syndrome : Results From the Randomized, Controlled MAESTRO Study
Read this article at
Abstract
Supplemental Digital Content is available in the text.
Abstract
Background:
Eisenmenger syndrome describes congenital heart disease-associated severe pulmonary hypertension accompanied by right-to-left shunting. The multicenter, double-blind, randomized, placebo-controlled, 16-week, phase III MAESTRO study (Macitentan in Eisenmenger Syndrome to Restore Exercise Capacity) evaluated the efficacy and safety of the endothelin receptor antagonist macitentan in patients with Eisenmenger syndrome.
Methods:
Patients with Eisenmenger syndrome aged ≥12 years and in World Health Organization functional class II–III were randomized 1:1 to placebo or macitentan 10 mg once daily for 16 weeks. Patients with complex cardiac defects, Down syndrome and background PAH therapy were eligible. The primary end point was change from baseline to week 16 in 6-minute walk distance. Secondary end points included change from baseline to week 16 in World Health Organization functional class. Exploratory end points included NT-proBNP (N-terminal pro-B-type natriuretic peptide) at end of treatment expressed as a percentage of baseline. In a hemodynamic substudy, exploratory end points included pulmonary vascular resistance index (PVRi) at week 16 as a percentage of baseline.
Results:
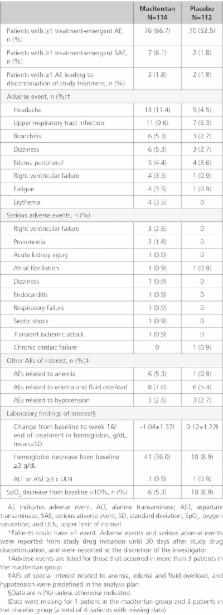
Two hundred twenty six patients (macitentan n=114; placebo n=112) were randomized. At baseline, 60% of patients were in World Health Organization functional class II and 27% were receiving phosphodiesterase type-5 inhibitors. At week 16, the mean change from baseline in 6-minute walk distance was 18.3 m and 19.7 m in the macitentan and placebo groups (least-squares mean difference, -4.7 m; 95% confidence limit (CL), -22.8, 13.5; P=0.612). World Health Organization functional class improved from baseline to week 16 in 8.8% and 14.3% of patients in the macitentan and placebo groups (odds ratio, 0.53; 95% CL, 0.23, 1.24). NT-proBNP levels decreased with macitentan versus placebo (ratio of geometric means, 0.80; 95% CL, 0.68, 0.94). In the hemodynamic substudy (n=39 patients), macitentan decreased PVRi compared with placebo (ratio of geometric means, 0.87; 95% CL, 0.73, 1.03). The most common adverse events with macitentan versus placebo were headache (11.4 versus 4.5%) and upper respiratory tract infection (9.6 versus 6.3%); a hemoglobin decrease from baseline of ≥2 g/dL occurred in 36.0% versus 8.9% of patients. Five patients (3 macitentan; 2 placebo) prematurely discontinued treatment and 1 patient died (macitentan group).
Related collections
Most cited references31
- Record: found
- Abstract: found
- Article: not found
Selexipag for the Treatment of Pulmonary Arterial Hypertension.
- Record: found
- Abstract: found
- Article: not found
Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2.
- Record: found
- Abstract: found
- Article: not found