- Record: found
- Abstract: found
- Article: found
SMYD2 promoter DNA methylation is associated with abdominal aortic aneurysm (AAA) and SMYD2 expression in vascular smooth muscle cells
Read this article at
Abstract
Background
Abdominal aortic aneurysm (AAA) is a deadly cardiovascular disease characterised by the gradual, irreversible dilation of the abdominal aorta. AAA is a complex genetic disease but little is known about the role of epigenetics. Our objective was to determine if global DNA methylation and CpG-specific methylation at known AAA risk loci is associated with AAA, and the functional effects of methylation changes.
Results
We assessed global methylation in peripheral blood mononuclear cell DNA from 92 individuals with AAA and 93 controls using enzyme-linked immunosorbent assays, identifying hyper-methylation in those with large AAA and a positive linear association with AAA diameter ( P < 0.0001, R 2 = 0.3175).
We then determined CpG methylation status of regulatory regions in genes located at AAA risk loci identified in genome-wide association studies, using bisulphite next-generation sequencing (NGS) in vascular smooth muscle cells (VSMCs) taken from aortic tissues of 44 individuals (24 AAAs and 20 controls). In IL6R, 2 CpGs were hyper-methylated ( P = 0.0145); in ERG, 13 CpGs were hyper-methylated ( P = 0.0005); in SERPINB9, 6 CpGs were hypo-methylated ( P = 0.0037) and 1 CpG was hyper-methylated ( P = 0.0098); and in SMYD2, 4 CpGs were hypo-methylated ( P = 0.0012).
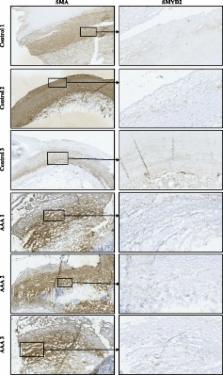
RT-qPCR was performed for each differentially methylated gene on mRNA from the same VSMCs and compared with methylation. This analysis revealed downregulation of SMYD2 and SERPINB9 in AAA, and a direct linear relationship between SMYD2 promoter methylation and SMYD2 expression ( P = 0.038). Furthermore, downregulation of SMYD2 at the site of aneurysm in the aortic wall was further corroborated in 6 of the same samples used for methylation and gene expression analysis with immunohistochemistry.
Conclusions
This study is the first to assess DNA methylation in VSMCs from individuals with AAA using NGS, and provides further evidence there is an epigenetic basis to AAA. Our study shows that methylation status of the SMYD2 promoter may be linked with decreased SMYD2 expression in disease pathobiology. In support of our work, downregulated SMYD2 has previously been associated with adverse cardiovascular physiology and inflammation, which are both hallmarks of AAA. The identification of such adverse epigenetic modifications could potentially contribute towards the development of epigenetic treatment strategies in the future.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Gene body methylation can alter gene expression and is a therapeutic target in cancer.
- Record: found
- Abstract: found
- Article: not found