- Record: found
- Abstract: found
- Article: found
Long noncoding RNA SNHG15 serves as an oncogene and predicts poor prognosis in epithelial ovarian cancer
Abstract
Objective
This study aims to investigate the functional role of long noncoding RNA SNHG15 in epithelial ovarian cancer (EOC).
Materials and methods
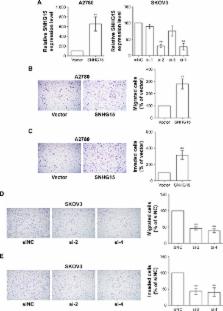
The expression of SNHG15 was measured in EOC cells and tissues using qRT-PCR. The correlation of SNHG15 expression and the clinicopathological characters was statistically analyzed. The prognosis of patients with different clinical features in the high/low SNHG15 expression groups were calculated. Moreover, univariate and multivariate Cox regression analyses were performed to identify the risk factors for poor overall survival (OS) and progression-free survival (PFS). The effect of SNHG15 on the migration and invasion was evaluated using Transwell and Matrigel, respectively. The proliferation ability of EOC cells was tested using colony formation and MTT assay. The influence of SNHG15 on the cisplatin resistance was detected by measuring cell inhibition rate and cell viability.
Results
SNHG15 was upegulated in EOC cells and tissues. High SNHG15 expression was correlated with EOC progression and predicted poor OS and PFS in different subgroups of EOC patients. Moreover, multivariate Cox regression analysis defined high SNHG15 expression as an independent risk factor for poor OS and PFS. Furthermore, functional assays showed that the overexpression of SNHG15 promoted migration and invasion, while the loss of SNHG15 suppressed migration and invasion. Furthermore, the proliferation of EOC cells was improved after the ectopic expression of SNHG15, which was suppressed with SNHG15 deficiency. In addition, cisplatin-resistant EOC cells were established for detecting the effect of SNHG15 on EOC chemoresistance. The results showed that cisplatin-resistant EOC cells exhibited much higher levels of SNHG15 expression than controls, and SNHG15 contributed to the chemoresistance of EOC cells.
Most cited references27
- Record: found
- Abstract: found
- Article: not found
The bright side of dark matter: lncRNAs in cancer.
- Record: found
- Abstract: found
- Article: not found
Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy.
- Record: found
- Abstract: found
- Article: not found