- Record: found
- Abstract: found
- Article: not found
Discovery of LRE1 as a specific and allosteric inhibitor of soluble adenylyl cyclase
Read this article at
Abstract
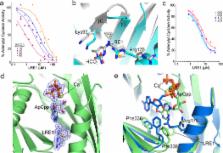
The prototypical second messenger cAMP regulates a wide variety of physiological processes. It can simultaneously mediate diverse functions by acting locally within independently-regulated microdomains. In mammalian cells, two types of adenylyl cyclase generate cAMP; G protein regulated transmembrane adenylyl cyclases and bicarbonate- calcium- and ATP-regulated soluble adenylyl cyclase (sAC). Because each type of cyclase regulates distinct microdomains, understanding cAMP signaling demands methods to distinguish between them. We developed a mass spectrometry based adenylyl cyclase assay which we used to identify a novel sAC-specific inhibitor, LRE1. LRE1 binds to the bicarbonate activator binding site and inhibits sAC via a unique allosteric mechanism. LRE1 prevents sAC-dependent processes in cellular and physiological systems and facilitates exploration of the therapeutic potential of sAC inhibition.
Related collections
Most cited references53
- Record: found
- Abstract: found
- Article: not found
Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor.
- Record: found
- Abstract: found
- Article: not found
Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells.
- Record: found
- Abstract: found
- Article: not found
