- Record: found
- Abstract: found
- Article: found
Phosphorylation of EB1 regulates the recruitment of CLIP-170 and p150 glued to the plus ends of astral microtubules

Read this article at
Abstract
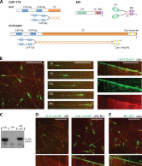
Phosphorylation of end-binding protein 1 (EB1), a key member of microtubule plus end-tracking proteins (+TIPs), by apoptosis signal-regulating kinase 1 (ASK1) has been demonstrated to promote the stability of astral microtubules during mitosis by stimulating the binding of EB1 to microtubule plus ends. However, the roles of other members of the +TIPs family in ASK1/EB1-mediated regulation of astral microtubules are unknown. Herein, we show that ASK1-mediated phosphorylation of EB1 enhances the localization of cytoplasmic linker protein 170 (CLIP-170) and p150 glued to the plus ends of astral microtubules. Depletion of ASK1 or expression of phospho-deficient or phospho-mimetic EB1 mutants results in changes in the levels of plus-end localized CLIP-170 or p150 glued. Mechanistic studies reveal that EB1 phosphorylation promotes its interactions with CLIP-170 and p150 glued, thereby recruiting these +TIPs to microtubules. Structural analysis suggests that serine-40 is the primary phosphorylation site on EB1 that exerts these effects. Together, these findings provide novel insight into the molecular mechanisms that regulate the interactions of EB1 with other +TIPs.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Control of microtubule organization and dynamics: two ends in the limelight.
- Record: found
- Abstract: found
- Article: not found
Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170.
- Record: found
- Abstract: found
- Article: found