- Record: found
- Abstract: found
- Article: found
Daily co-trimoxazole prophylaxis to prevent mortality in children with complicated severe acute malnutrition: a multicentre, double-blind, randomised placebo-controlled trial
Summary
Background
Children with complicated severe acute malnutrition (SAM) have a greatly increased risk of mortality from infections while in hospital and after discharge. In HIV-infected children, mortality and admission to hospital are prevented by daily co-trimoxazole prophylaxis, despite locally reported bacterial resistance to co-trimoxazole. We aimed to assess the efficacy of daily co-trimoxazole prophylaxis on survival in children without HIV being treated for complicated SAM.
Methods
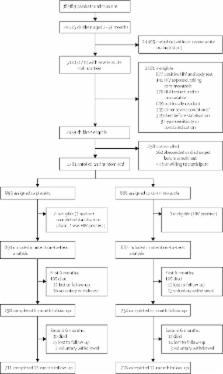
We did a multicentre, double-blind, randomised, placebo-controlled study in four hospitals in Kenya (two rural hospitals in Kilifi and Malindi, and two urban hospitals in Mombasa and Nairobi) with children aged 60 days to 59 months without HIV admitted to hospital and diagnosed with SAM. We randomly assigned eligible participants (1:1) to 6 months of either daily oral co-trimoxazole prophylaxis (given as water-dispersible tablets; 120 mg per day for age <6 months, 240 mg per day for age 6 months to 5 years) or matching placebo. Assignment was done with computer-generated randomisation in permuted blocks of 20, stratified by centre and age younger or older than 6 months. Treatment allocation was concealed in opaque, sealed envelopes and patients, their families, and all trial staff were masked to treatment assignment. Children were given recommended medical care and feeding, and followed up for 12 months. The primary endpoint was mortality, assessed each month for the first 6 months, then every 2 months for the second 6 months. Secondary endpoints were nutritional recovery, readmission to hospital, and illness episodes treated as an outpatient. Analysis was by intention to treat. This trial was registered at ClinicalTrials.gov, number NCT00934492.
Findings
Between Nov 20, 2009, and March 14, 2013, we recruited and assigned 1778 eligible children to treatment (887 to co-trimoxazole prophylaxis and 891 to placebo). Median age was 11 months (IQR 7–16 months), 306 (17%) were younger than 6 months, 300 (17%) had oedematous malnutrition (kwashiorkor), and 1221 (69%) were stunted (length-for-age Z score <–2). During 1527 child-years of observation, 122 (14%) of 887 children in the co-trimoxazole group died, compared with 135 (15%) of 891 in the placebo group (unadjusted hazard ratio [HR] 0·90, 95% CI 0·71–1·16, p=0·429; 16·0 vs 17·7 events per 100 child-years observed (CYO); difference −1·7 events per 100 CYO, 95% CI −5·8 to 2·4]). In the first 6 months of the study (while participants received study medication), 63 suspected grade 3 or 4 associated adverse events were recorded among 57 (3%) children; 31 (2%) in the co-trimoxazole group and 32 (2%) in the placebo group (incidence rate ratio 0·98, 95% CI 0·58–1·65). The most common adverse events of these grades were urticarial rash (grade 3, equally common in both groups), neutropenia (grade 4, more common in the co-trimoxazole group), and anaemia (both grades equally common in both groups). One child in the placebo group had fatal toxic epidermal necrolysis with concurrent Pseudomonas aeruginosa bacteraemia.
Related collections
Most cited references6
- Record: found
- Abstract: found
- Article: not found
Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost?
- Record: found
- Abstract: found
- Article: not found
Viral etiology of severe pneumonia among Kenyan infants and children.
- Record: found
- Abstract: found
- Article: not found