- Record: found
- Abstract: found
- Article: not found
Identification of novel compounds against three targets of SARS CoV-2 coronavirus by combined virtual screening and supervised machine learning
Read this article at
Abstract
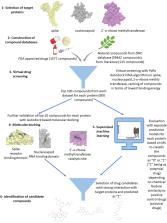
Coronavirus disease 2019 (COVID-19) is a major threat worldwide due to its fast spreading. As yet, there are no established drugs or vaccines available. Speeding up drug discovery is urgently required. We applied a workflow of combined in silico methods (virtual drug screening, molecular docking and supervised machine learning algorithms) to identify novel drug candidates against COVID-19. We constructed chemical libraries consisting of FDA-approved drugs for drug repositioning and of natural compound datasets from literature mining and the ZINC database to select compounds interacting with SARS-CoV-2 target proteins (spike protein, nucleocapsid protein, and 2’-o-ribose methyltransferase). Supported by the supercomputer MOGON, candidate compounds were predicted as presumable SARS-CoV-2 inhibitors. Interestingly, several approved drugs against hepatitis C virus (HCV), another enveloped (-) ssRNA virus (paritaprevir, simeprevir and velpatasvir) as well as drugs against transmissible diseases, against cancer, or other diseases were identified as candidates against SARS-CoV-2. This result is supported by reports that anti-HCV compounds are also active against Middle East Respiratory Virus Syndrome (MERS) coronavirus. The candidate compounds identified by us may help to speed up the drug development against SARS-CoV-2.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: found
Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China

- Record: found
- Abstract: found
- Article: found
A new coronavirus associated with human respiratory disease in China
- Record: found
- Abstract: found
- Article: not found
