- Record: found
- Abstract: found
- Article: found
Changes in the asymmetric distribution of cholesterol in the plasma membrane influence streptolysin O pore formation
Read this article at
Abstract
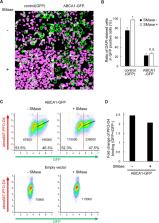
ATP-binding cassette A1 (ABCA1) plays a key role in generating high-density lipoprotein (HDL) and preventing atherosclerosis. ABCA1 exports cholesterol and phospholipid to apolipoprotein A-I (apoA-I) in serum to generate HDL. We found that streptolysin O (SLO), a cholesterol-dependent pore-forming toxin, barely formed pores in ABCA1-expressing cells, even in the absence of apoA-I. Neither cholesterol content in cell membranes nor the amount of SLO bound to cells was affected by ABCA1. On the other hand, binding of the D4 domain of perfringolysin O (PFO) to ABCA1-expressing cells increased, suggesting that the amount of cholesterol in the outer leaflet of the plasma membrane (PM) increased and that the cholesterol dependences of these two toxins differ. Addition of cholesterol to the PM by the MβCD–cholesterol complex dramatically restored SLO pore formation in ABCA1-expressing cells. Therefore, exogenous expression of ABCA1 causes reduction in the cholesterol level in the inner leaflet, thereby suppressing SLO pore formation.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Lipid rafts: at a crossroad between cell biology and physics.
- Record: found
- Abstract: found
- Article: not found
Tangier disease is caused by mutations in the gene encoding ATP-binding cassette transporter 1.
- Record: found
- Abstract: not found
- Article: not found