- Record: found
- Abstract: found
- Article: found
Distinct contributions of the thin and thick filaments to length-dependent activation in heart muscle
Read this article at
Abstract
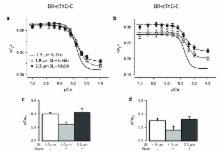
The Frank-Starling relation is a fundamental auto-regulatory property of the heart that ensures the volume of blood ejected in each heartbeat is matched to the extent of venous filling. At the cellular level, heart muscle cells generate higher force when stretched, but despite intense efforts the underlying molecular mechanism remains unknown. We applied a fluorescence-based method, which reports structural changes separately in the thick and thin filaments of rat cardiac muscle, to elucidate that mechanism. The distinct structural changes of troponin C in the thin filaments and myosin regulatory light chain in the thick filaments allowed us to identify two aspects of the Frank-Starling relation. Our results show that the enhanced force observed when heart muscle cells are maximally activated by calcium is due to a change in thick filament structure, but the increase in calcium sensitivity at lower calcium levels is due to a change in thin filament structure.
eLife digest
The heart needs to pump out the same volume of blood that enters it. This is not as simple as it sounds, as changes in heart rate – for example, in response to exercise – alter how hard the heart must pump.
When blood flows into the heart it stretches the heart muscle, which consists of units called sarcomeres. Sarcomeres contain two types of protein filament, known as thick filaments and thin filaments. When a heartbeat is triggered by calcium ions flowing into the heart muscle cells, the thick filaments slide over the thin filaments. This causes the heart muscle cell to contract.
The Frank–Starling mechanism helps to regulate the contraction of the heart. This mechanism has two aspects. Firstly, as the sarcomere lengthens, its protein filaments are able to contract with more force for a given high level of calcium ions. Secondly, the lengthening of the sarcomere makes the filaments more sensitive to calcium ions, which again causes the heart to contract more forcefully. However, the molecular mechanisms that underlie these effects were not clear.
Zhang et al. have now studied rat heart muscle cells using a new fluorescence-based method that can detect structural changes in the thick and thin filaments. The results show that the increased force that is generated when sarcomeres are stretched can be accounted for by changes in the structure of the thick filament. In contrast, the increase in calcium sensitivity that occurs as the sarcomere lengthens is largely due to structural alterations in the thin filament. These two processes can be controlled independently, but work together in the Frank–Starling mechanism.
Now that we better understand the molecular basis of the Frank–Starling mechanism, further work could investigate new strategies for designing and testing treatments for heart disease.
Related collections
Most cited references57
- Record: found
- Abstract: found
- Article: not found
Regulation of contraction in striated muscle.
- Record: found
- Abstract: found
- Article: not found
Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form.
- Record: found
- Abstract: found
- Article: not found