- Record: found
- Abstract: found
- Article: found
Biomaterials: Foreign Bodies or Tuners for the Immune Response?
Read this article at
Abstract
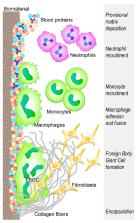
The perspectives of regenerative medicine are still severely hampered by the host response to biomaterial implantation, despite the robustness of technologies that hold the promise to recover the functionality of damaged organs and tissues. In this scenario, the cellular and molecular events that decide on implant success and tissue regeneration are played at the interface between the foreign body and the host inflammation, determined by innate and adaptive immune responses. To avoid adverse events, rather than the use of inert scaffolds, current state of the art points to the use of immunomodulatory biomaterials and their knowledge-based use to reduce neutrophil activation, and optimize M1 to M2 macrophage polarization, Th1 to Th2 lymphocyte switch, and Treg induction. Despite the fact that the field is still evolving and much remains to be accomplished, recent research breakthroughs have provided a broader insight on the correct choice of biomaterial physicochemical modifications to tune the reaction of the host immune system to implanted biomaterial and to favor integration and healing.
Related collections
Most cited references311
- Record: found
- Abstract: found
- Article: not found
The microbiome and innate immunity.
- Record: found
- Abstract: found
- Article: not found