- Record: found
- Abstract: found
- Article: found
Insulin signaling and the regulation of insect diapause
Read this article at
Abstract
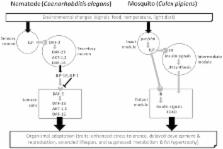
A rich chapter in the history of insect endocrinology has focused on hormonal control of diapause, especially the major roles played by juvenile hormones (JHs), ecdysteroids, and the neuropeptides that govern JH and ecdysteroid synthesis. More recently, experiments with adult diapause in Drosophila melanogaster and the mosquito Culex pipiens, and pupal diapause in the flesh fly Sarcophaga crassipalpis provide strong evidence that insulin signaling is also an important component of the regulatory pathway leading to the diapause phenotype. Insects produce many different insulin-like peptides (ILPs), and not all are involved in the diapause response; ILP-1 appears to be the one most closely linked to diapause in C. pipiens. Many steps in the pathway leading from perception of daylength (the primary environmental cue used to program diapause) to generation of the diapause phenotype remain unknown, but the role for insulin signaling in mosquito diapause appears to be upstream of JH, as evidenced by the fact that application of exogenous JH can rescue the effects of knocking down expression of ILP-1 or the Insulin Receptor. Fat accumulation, enhancement of stress tolerance, and other features of the diapause phenotype are likely linked to the insulin pathway through the action of a key transcription factor, FOXO. This review highlights many parallels for the role of insulin signaling as a regulator in insect diapause and dauer formation in the nematode Caenorhabditis elegans.
Related collections
Most cited references117
- Record: found
- Abstract: found
- Article: not found
daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans.
- Record: found
- Abstract: found
- Article: not found
Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway.
- Record: found
- Abstract: found
- Article: not found