- Record: found
- Abstract: found
- Article: found
A randomized controlled phase IIb wound healing trial of cutaneous leishmaniasis ulcers with 0.045% pharmaceutical chlorite (DAC N-055) with and without bipolar high frequency electro-cauterization versus intralesional antimony in Afghanistan
Read this article at
Abstract
Background
A previously published proof of principle phase IIa trial with 113 patients from Kabul showed that bipolar high-frequency (HF) electro-cauterization (EC) of cutaneous leishmaniasis (CL) ulcers and subsequent moist wound treatment (MWT) closed 85% of all Leishmania (L.) tropica lesions within 60 days.
Methods
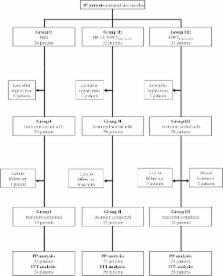
A three-armed phase IIb, randomized and controlled clinical trial was performed in Mazar-e-Sharif. L. tropica- or L. major-infected CL patients received intradermal sodium stibogluconate (SSG) (Group I); HF-EC followed by MWT with 0.045% DAC N-055 (Group II); or MWT with 0.045% DAC N-055 in basic crème alone (Group III). The primary outcome was complete epithelialisation before day 75 after treatment start.
Results
87 patients enrolled in the trial were randomized into group I (n = 24), II (n = 32) and III (n = 31). The per-protocol analysis of 69 (79%) patients revealed complete epithelialisation before day 75 in 15 (of 23; 65%) patients of Group I, in 23 (of 23; 100%) patients of Group II, and in 20 (of 23; 87%) patients of Group III (p = 0.004, Fisher’s Exact Test). In the per-protocol analysis, wound closure times were significantly different between all regimens in a pair-wise comparison (p = 0.000039, Log-Rank (Mantel-Cox) test). In the intention-to-treat analysis wound survival times in Group II were significantly different from those in Group I (p = 0.000040, Log-Rank (Mantel-Cox) test). Re-ulcerations occurred in four (17%), three (13%) and seven (30%) patients of Group I, II or III, respectively (p = 0.312, Pearson Chi-Square Test).
Conclusions
Treatment of CL ulcers with bipolar HF-EC followed by MWT with 0.045% DAC N-055 or with DAC N-055 alone showed shorter wound closure times than with the standard SSG therapy. The results merit further exploration in larger trials in the light of our current knowledge of in vitro and in vivo activities of chlorite. Clinicaltrials.gov ID: NCT00996463. Registered: 15 th October 2009.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients.
- Record: found
- Abstract: found
- Article: not found
A review of the scientific evidence for biofilms in wounds.

- Record: found
- Abstract: found
- Article: found