- Record: found
- Abstract: found
- Article: found
Serum hepatitis B surface antigen titer and transient elastography in screening for insignificant fibrosis in HBeAg-positive chronic hepatitis B patients
Abstract
Objective
To explore the predictive value of serum hepatitis B surface antigen (HBsAg) titer and transient elastography in screening for insignificant fibrosis in hepatitis B e antigen (HBeAg)-positive chronic hepatitis B patients.
Methods
We conducted a cross-sectional study of eligible patients treated from March 2012 to May 2013 at the West China Hospital of Sichuan University. Eligible patients underwent liver transient elastography and liver biopsy. We assessed the serum HBsAg level, serum hepatitis B virus (HBV) deoxyribonucleic acid (DNA) level, HBV genotypes, liver stiffness measurement (LSM) values by transient elastography, and histological fibrosis staging by METAVIR classification.
Results
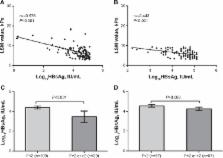
A total of 129 consecutive patients were recruited. The LSM value ( P<0.001, odds ratio 14.67, 95% CI 0.158–0.551) and log 10HBsAg ( P=0.045, odds ratio 4.03, 95% CI 0.136–0.976) correlated with a liver fibrosis score <F2, independently. Inverse correlations were found between log 10HBsAg and the LSM value ( r=−576, P<0.001) and fibrosis staging ( r=−374, P<0.001). Patients with a fibrosis score <F2 had a significantly higher log 10HBsAg than patients with a fibrosis score ≥F2 among those with an LSM value under 9.4 kPa (4.6±0.7 vs 4.3±0.5, P=0.006). The HBsAg titer achieved an area under the receiver operating characteristic curve of 0.758 ( P<0.001, 95% CI 0.631–0.884) in predicting a fibrosis score <F2, with a cut-off value of 10,400 IU/mL, a positive predictive value of 73%, and a negative predictive value of 79%.
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Global control of hepatitis B virus infection.
- Record: found
- Abstract: found
- Article: not found
Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study).
- Record: found
- Abstract: found
- Article: not found