- Record: found
- Abstract: found
- Article: found
Understanding the Role of Maternal Diet on Kidney Development; an Opportunity to Improve Cardiovascular and Renal Health for Future Generations
Read this article at
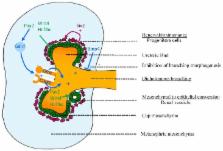
Abstract
The leading causes of mortality and morbidity worldwide are cardiovascular disease (high blood pressure, high cholesterol and renal disease), cancer and diabetes. It is increasingly obvious that the development of these diseases encompasses complex interactions between adult lifestyle and genetic predisposition. Maternal malnutrition can influence the fetal and early life environment and pose a risk factor for the future development of adult diseases, most likely due to impaired organogenesis in the developing offspring. This then predisposes these offspring to cardiovascular disease and renal dysfunction in adulthood. Studies in experimental animals have further illustrated the significant impact maternal diet has on offspring health. Many studies report changes in kidney structure (a reduction in the number of nephrons in the kidney) in offspring of protein-deprived dams. Although the early studies suggested that increased blood pressure was also present in offspring of protein-restricted dams, this is not a universal finding and requires clarification. Importantly, to date, the literature offers little to no understanding of when in development these changes in kidney development occur, nor are the cellular and molecular mechanisms that drive these changes well characterised. Moreover, the mechanisms linking maternal nutrition and a suboptimal renal phenotype in offspring are yet to be discerned—one potential mechanism involves epigenetics. This review will focus on recent information on potential mechanisms by which maternal nutrition (focusing on malnutrition due to protein restriction, micronutrient restriction and excessive fat intake) influences kidney development and thereby function in later life.
Related collections
Most cited references147
- Record: found
- Abstract: found
- Article: not found
Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development.
- Record: found
- Abstract: found
- Article: not found
Nephron number in patients with primary hypertension.
- Record: found
- Abstract: found
- Article: not found